How Much Vitamin C Can Cause Kidney Stones
Contents
- Summary
- Function
- Role in immunity
- Bioavailability
- Deficiency
- The RDA
- Disease Prevention
- Cardiovascular disease
- Cancer
- Type 2 diabetes mellitus
- Adverse pregnancy outcomes
- Alzheimer's disease
- Cataracts
- Gout
- Mortality
- Disease Treatment
- Cardiovascular disease
- Sepsis
- Cancer
- Common cold
- Asthma
- Lead toxicity
- Sources
- Food sources
- Supplements
- Safety
- Toxicity
- Kidney stones
- Drug interactions
- Pro-oxidant effects
- LPI Recommendation
- Authors and Reviewers
- References
Español
Summary
- Vitamin C, also known as L-ascorbic acid, is a water-soluble vitamin. Unlike most mammals and other animals, humans do not have the ability to synthesize vitamin C and must obtain it from the diet. (More information)
- Vitamin C is an essential cofactor in numerous enzymatic reactions, e.g., in the biosynthesis of collagen, carnitine, and neuropeptides, and in the regulation of gene expression. It is also a potent antioxidant. (More information)
- Prospective cohort studies indicate that higher vitamin C status, assessed by measuring circulating vitamin C, is associated with lower risks of hypertension, coronary heart disease, and stroke. (More information)
- There is some evidence to suggest that vitamin C may be a useful adjunct to conventional medical practice to reduce myocardial injury and arrhythmia following a cardiac procedure or surgery in patients with cardiovascular disease.(More information)
- There are insufficient data to suggest a link between vitamin C status and the risk of developing a given type of cancer. Most observational studies examining vitamin C intake in relation to cancer incidence have found no association. Randomized controlled trials have reported no effect of vitamin C supplementation on cancer risk.(More information)
- Current evidence of the efficacy of intravenous vitamin C in cancer patients is limited to observational studies, uncontrolled interventions, and case reports. There is a need for large, longer-duration phase II clinical trials that test the efficacy of intravenous vitamin C in cancer progression and overall survival.(More information)
- Overall, regular use of vitamin C supplements shortens the duration of the common cold but does not reduce the risk of becoming ill. Taking supplements once cold symptoms have already begun has no proven benefits.(More information)
- Vitamin C supplements are available in many forms, but there is little scientific evidence that any one form is better absorbed or more effective than another. (More information)
- There is no scientific evidence that large amounts of vitamin C (up to 10 grams [g]/day in adults) exert any adverse or toxic effects. An upper intake level of 2 g/day is recommended in order to prevent some adults from experiencing diarrhea and gastrointestinal disturbances.(More information)
- Supplemental vitamin C increases urinary oxalate concentrations, but whether an increase in urinary oxalate elevates the risk for kidney stones is not yet known. Those predisposed for kidney stone formation may consider avoiding high-dose (≥1 g/day) vitamin C supplementation.(More information)
Function
Vitamin C (L-ascorbic acid) is a potent reducing agent, meaning that it readily donates electrons to recipient molecules (Figure 1). Related to this oxidation-reduction (redox) potential, two major functions of vitamin C are as an antioxidant and as an enzyme cofactor (1).
Vitamin C is the primary water-soluble, non-enzymatic antioxidant in plasma and tissues. Even in small amounts, vitamin C can protect indispensable molecules in the body, such as proteins, lipids (fats), carbohydrates, and nucleic acids (DNA and RNA), from damage by free radicals and reactive oxygen species (ROS) that are generated during normal metabolism, by active immune cells, and through exposure to toxins and pollutants (e.g., certain chemotherapy drugs and cigarette smoke). Vitamin C also participates in redox recycling of other important antioxidants; for example, vitamin C is known to regenerate vitamin E from its oxidized form (see the article on Vitamin E).
The role of vitamin C as a cofactor is also related to its redox potential. By maintaining enzyme-bound metals in their reduced forms, vitamin C assists mixed-function oxidases in the synthesis of several critical biomolecules (1). These enzymes are either monooxygenases or dioxygenases (see Table 1). Symptoms of vitamin C deficiency, such as poor wound healing and lethargy, likely result from the impairment of these vitamin C-dependent enzymatic reactions leading to the insufficient synthesis of collagen, carnitine, and catecholamines (see Deficiency). Moreover, several dioxygenases involved in the regulation of gene expression and the maintenance of genome integrity require vitamin C as a cofactor. Indeed, research has recently uncovered the crucial role played by enzymes, such as the TET dioxygenases and Jumonji domain-containing histone demethylases, in the fate of cells and tissues (seeTable 1). These enzymes contribute to the epigenetic regulation of gene expression by catalyzing reactions involved in the demethylation of DNA and histones.
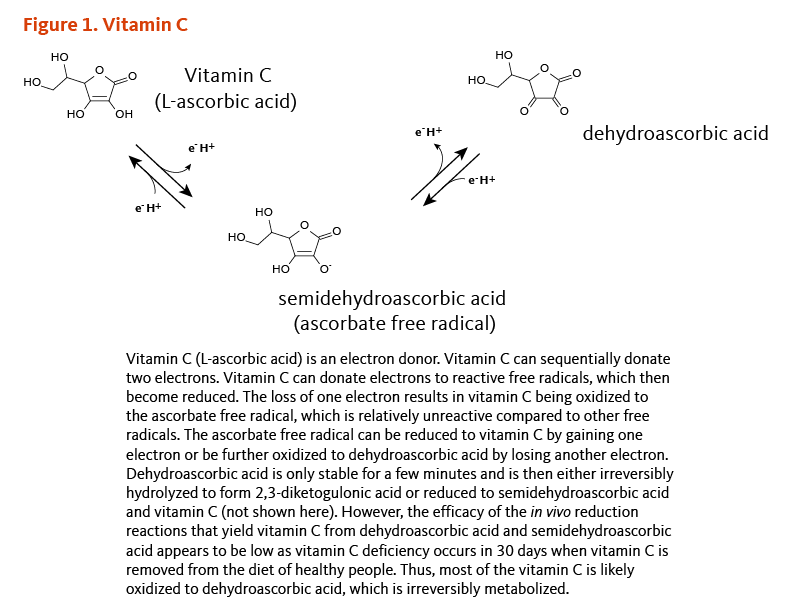
[Figure 1 - Click to Enlarge]
| Enzymes* | Functions |
|---|---|
| Monooxygenases | |
| Dopamine β-monooxygenase | Norepinephrine (Noradrenaline) biosynthesis |
| Peptidyl-glycine α-amidating monooxygenase | Amidation of peptide hormones |
| Dioxygenases | |
| 3 Prolyl 4-hydroxylase isoenzymes | Collagen hydroxylation |
| 3 Prolyl 3-hydroxylase isoenzymes | Collagen hydroxylation |
| 3 Lysyl hydroxylase isoenzymes | Collagen hydroxylation |
| 4 Hypoxia-inducible factor (HIF) isoenzymes | HIF hydroxylation |
| Trimethyllysine hydroxylase | Carnitine biosynthesis |
| γ-Butyrobetaine hydroxylase | Carnitine biosynthesis |
| 4-Hydroxyphenylpyruvate dioxygenase | Tyrosine metabolism |
| Ten-eleven translocation (TET) family of dioxygenases | Demethylation of DNA |
| Jumonji domain-containing histone demethylases | Demethylation of histones |
| *Monooxygenases catalyze the hydroxylation of one substrate, whereas dioxygenases catalyze a reaction that couples the hydroxylation of a specific substrate with the conversion (decarboxylation) of α-ketoglutarate into succinate. | |
The capacity of vitamin C to influence the methylation status of DNA and histones in mammalian cells supports a role for the vitamin in health and disease beyond what was previously understood, in particular by safeguarding genome integrity (3, 4).
Role in immunity
Vitamin C affects several components of the human immune system in vitro; for example, vitamin C has been shown to stimulate both the production (5-9) and function (10, 11) of leukocytes (white blood cells), especially neutrophils, lymphocytes, and phagocytes. Specific measures of functions stimulated by vitamin C include cellular motility (10), chemotaxis (10, 11), and phagocytosis (11). Neutrophils, mononuclear phagocytes, and lymphocytes accumulate vitamin C to high concentrations, which can protect these cell types from oxidative damage (12-14). In response to invading microorganisms, phagocytic leukocytes release non-specific toxins, such as superoxide radicals, hypochlorous acid ("bleach"), and peroxynitrite; these reactive oxygen species kill pathogens and, in the process, can damage the leukocytes themselves (15). Vitamin C, through its antioxidant functions, has been shown to protect leukocytes from self-inflicted oxidative damage (14). Phagocytic leukocytes also produce and release cytokines, including interferons, which have antiviral activity (16). Vitamin C has been shown to increase interferon production in vitro (17). Additional studies have reported that vitamin C enhances the chemotactic and microbial killing capacities of neutrophils and stimulates the proliferation and differentiation of B- and T-lymphocytes (reviewed in 18).
It is widely thought by the general public that vitamin C boosts immune function, yet human studies published to date are conflicting. Disparate results are likely due to study design issues, often linked to a lack of understanding of vitamin C pharmacokinetics and requirements (19, 20).
Finally, vitamin C increases the bioavailability of iron from foods by enhancing intestinal absorption of non-heme iron (see the article on Iron) (21).
Bioavailability
Depletion-repletion pharmacokinetic experiments demonstrated that plasma vitamin C concentration is tightly controlled by three primary mechanisms: intestinal absorption, tissue transport, and renal reabsorption (22). In response to increasing oral doses of vitamin C, plasma vitamin C concentration rises steeply at intakes between 30 and 100 mg/day. Plasma concentrations of ascorbate reach steady-state at concentrations between 60 and 80 micromoles/L (μmol/L). This is typically observed at doses between 200 to 400 mg/day in healthy young adults, with some degree of individual variation (23, 24).
One hundred percent absorption efficiency is observed when ingesting vitamin C at doses up to 200 mg at a time. Higher doses (>500 mg) result in fractionally less vitamin C being absorbed as the dose increases. Once plasma vitamin C concentrations reach saturation, additional vitamin C is largely excreted in the urine. Notably, intravenous administration of vitamin C bypasses absorptive control in the intestine such that very high concentrations of vitamin C can be achieved in the plasma; within a few hours, renal excretion restores vitamin C to baseline plasma concentrations (see Cancer Treatment) (25).
While plasma vitamin C concentration reflects recent dietary intake, leukocyte (white blood cell) vitamin C is thought to be an indicator of body stores. However, leukocyte vitamin C concentration does not accurately reflect vitamin C in several tissues and may specifically underestimate vitamin C uptake into skeletal muscle (26). Yet, plasma concentrations of vitamin C ≥50 μmol/L are sufficient to saturate muscle tissue vitamin C.
There is also some limited evidence suggesting that individuals who carry certain polymorphisms in genes involved in vitamin C transport and detoxification mechanisms may have lower plasma vitamin C concentrations even with high vitamin C intakes (see also Vascular complications of diabetes mellitus) (reviewed in 27).
Due to the pharmacokinetics and tight regulation of plasma vitamin C, supplementation with vitamin C will have variable effects in vitamin C-replete (plasma concentrations near saturation) versus sub-optimal (plasma concentrations <50 μmol/L), marginally deficient (plasma concentrations <23 μmol/L), or severely deficient (plasma concentrations <11 μmol/L) individuals (28). Scientific studies investigating vitamin C efficacy to prevent or treat disease need to assess baseline vitamin C status before embarking on an intervention or statistical analysis (22, 29-31).
For a more detailed discussion on the bioavailability of different forms of vitamin C, see the article, The Bioavailability of Different Forms of Vitamin C.
Deficiency
Severe vitamin C deficiency has been known for many centuries as the potentially fatal disease, scurvy. By the late 1700s, the British navy was aware that scurvy could be cured by eating oranges or lemons, even though vitamin C would not be isolated until the early 1930s. Symptoms of scurvy include subcutaneous bleeding, poor wound closure, and bruising easily, hair and tooth loss, and joint pain and swelling. Such symptoms appear to be related to the weakening of blood vessels, connective tissue, and bone, which all contain collagen. Early symptoms of scurvy like fatigue may result from diminished levels of carnitine, which is needed to derive energy from fat, or from decreased synthesis of the catecholamine norepinephrine (see Function). Scurvy is rare in developed countries because it can be prevented by as little as 10 mg of vitamin C daily (32). However, cases have occurred in children and the elderly on very restricted diets (33, 34).
The Recommended Dietary Allowance (RDA)
The recommended dietary allowance (RDA) for vitamin C is based on the amount of vitamin C intake necessary to maintain neutrophil vitamin C concentration with minimal urinary excretion of vitamin C and is proposed to provide sufficient antioxidant protection (Table 2) (35). The recommended intake for smokers is 35 mg/day higher than for nonsmokers, because smokers are under increased oxidative stress from the toxins in cigarette smoke and generally have lower blood concentrations of vitamin C (36).
| Life Stage | Age | Males (mg/day) | Females (mg/day) |
|---|---|---|---|
| Infants | 0-6 months | 40 (AI) | 40 (AI) |
| Infants | 7-12 months | 50 (AI) | 50 (AI) |
| Children | 1-3 years | 15 | 15 |
| Children | 4-8 years | 25 | 25 |
| Children | 9-13 years | 45 | 45 |
| Adolescents | 14-18 years | 75 | 65 |
| Adults | 19 years and older | 90 | 75 |
| Smokers | 19 years and older | 125 | 110 |
| Pregnancy | 18 years and younger | - | 80 |
| Pregnancy | 19 years and older | - | 85 |
| Breast-feeding | 18 years and younger | - | 115 |
| Breast-feeding | 19 years and older | - | 120 |
Disease Prevention
The amount of vitamin C required to help prevent chronic disease is higher than the amount required for prevention of scurvy. Information regarding vitamin C and the prevention of chronic disease is based on both observational prospective cohort studies and randomized controlled trials (29, 37). Prospective cohort studies can examine the incidence of a specific disease in relation to vitamin C intake or body status in a cohort of participants who are followed over time. In contrast, trials are intervention studies that can establish a causal relationship between an exposure and an outcome, e.g., by evaluating the effect of vitamin C supplementation on the incidence of chronic disease in participants randomly assigned to receive either vitamin C or placebo for a given length of time.
Cardiovascular disease
Endothelial dysfunction
Endothelial dysfunction is considered to be an early step in the development of atherosclerosis. Alterations in the structure and function of the vascular endothelium that lines the inner surface of all blood vessels are associated with the loss of normal nitric oxide-mediated endothelium-dependent vasodilation. Endothelial dysfunction results in widespread vasoconstriction and coagulation abnormalities. The measurement of brachial artery flow-mediated dilation (FMD) is often used as a functional marker of endothelial function; FMD values are inversely correlated with the risk of future cardiovascular events (38). A 2014 meta-analysis of 44 randomized controlled trials in subjects with or without chronic diseases summarized the effect of supplemental vitamin C on endothelial function by measuring FMD (19 studies), assessing forearm blood flow (20 studies), or by pulse wave analysis (5 trials) (39). Short-term supplementation with vitamin C was found to reduce endothelial dysfunction in subjects with heart failure, atherosclerosis, or diabetes mellitus, but it had no effect in those with hypertension. Vitamin C also limited endothelial dysfunction that was experimentally induced in healthy volunteers (39). Improved endothelial function was observed with daily vitamin C doses above 500 mg (39).
Hypertension
Hypertension is a major risk factor for cardiovascular disease, including coronary heart disease, stroke, and atrial fibrillation. An analysis that combined data from three, large, independent prospective cohorts, (1) Nurses' Health Study 1 (NHS1; 88,540 women, median age 49 years); (2) Nurses' Health Study 2 (NHS2; 97,315 women, median age 36 years); and (3) Health Professionals Follow-up Study (HPFS; 37,375 men, median age 52 years), found no association between the level of vitamin C intake and risk of developing hypertension (40). On the other hand, when plasma vitamin C concentration was measured, cross-sectional studies have consistently indicated an inverse relationship between plasma vitamin C concentration and blood pressure in both men and women (41-43). A 15-year follow-up of about 2,500 participants in the Coronary Artery Risk Development in Young Adults (CARDIA) study found that higher plasma vitamin C and a higher diet quality score were independently associated with a reduced risk of developing hypertension (44). Interestingly, there was no relationship between diet score and risk of hypertension in those with the lowest plasma vitamin C, and plasma vitamin C was positively associated with risk of hypertension in those with low diet scores (44).
A meta-analysis of 29 small randomized controlled trials of short durations (median duration, 8 weeks) in 1,407 participants (10 to 120 subjects per trial; including both normotensive and hypertensive subjects) found that daily supplementation with 60 to 4,000 mg of vitamin C (median dose, 500 mg) reduced systolic blood pressure by 3.84 mm Hg and diastolic blood pressure by 1.48 mm Hg (45). Good quality long-term trials are needed to examine whether the anti-hypertensive effect of vitamin C is sustained over time and eventually results in a reduced risk of cardiovascular events.
It is important for individuals with significantly elevated blood pressure not to rely on vitamin C supplementation alone to reduce their hypertension. They should instead seek or continue treatment with anti-hypertensive medication and make dietary and lifestyle changes in consultation with their health care provider.
Cardiovascular disease risk
Coronary heart disease (CHD) is characterized by the buildup of plaque inside the arteries that supply blood to the heart (atherosclerosis). Over years of buildup and accumulated damage to the coronary arteries, CHD may culminate in a myocardial infarction or heart attack. Many prospective cohort studies have examined the relationship between vitamin C intake from diet and supplements and CHD risk, the results of which have been pooled and analyzed in two separate analyses (46, 47). In 2004, a pooled analysis of nine prospective cohort studies found that supplemental vitamin C intake (≥400 mg/day for a mean of 10 years), but not dietary vitamin C intake, was inversely associated with CHD risk (46). Conversely, a 2008 meta-analysis of 14 cohort studies concluded that dietary, but not supplemental, vitamin C intake was inversely related to CHD risk (47). The most recent large prospective cohort study found an inverse association between dietary vitamin C intake and CHD mortality in Japanese women, but not in men (48). In spite of the variable association depending on source, these analyses indicate an overall inverse association between higher vitamin C intakes and CHD risk.
Limitations inherent to dietary assessment methodology, such as recall bias, measurement error, and residual confounding, may account for some of the inconsistent associations between vitamin C intake and CHD risk. In order to overcome such limitations, some prospective studies measured plasma or serum concentrations of vitamin C as a more reliable index of vitamin C intake and biomarker of body vitamin C status.
The European Investigation into Cancer and Nutrition (EPIC)-Norfolk prospective cohort study investigated the relationship between vitamin C status and incident heart failure in healthy adults (9,187 men and 11,112 women, aged 58.1+/-9.2 years) (49). After a mean follow-up of 12.8 years, plasma vitamin C was inversely associated with incident cases of heart failure. Specifically, plasma vitamin C ranged from approximately 23 to 70 μmol/L in men and 33 to 82 μmol/L in women; across this range, every 20 μmol/L increase in plasma vitamin C was associated with a 9% reduction in risk of heart failure. Although a primary source of dietary vitamin C, consumption of fruit and vegetables — assessed by food frequency questionnaire — was not found to be associated with a lower risk of congestive heart failure (49). This highlights the fact that limitations associated with dietary assessment methods such as food frequency questionnaires may be overcome by using biomarkers of nutrient intake (50, 51).
A 2017 review of eight published randomized controlled trials found inconsistent results from seven trials reporting on the effect of vitamin C supplementation on serum cholesterol and triglycerides, established risk factors for cardiovascular disease (52). Only one large trial in more than 14,000 older men participating in the Physicians' Health Study II (PHS II) reported on cardiovascular outcomes. PHS II found that vitamin C supplementation (500 mg/day) for an average of eight years had no significant effect on major cardiovascular events, total myocardial infarction, or cardiovascular mortality (53). Notably, this study had several limitations (54), including no measurement of vitamin C status and the recruitment of a well-nourished study population.
There is a need for better quality studies to examine the effect of vitamin C on cardiovascular endpoints in participants with elevated risk of cardiovascular disease.
Stroke
A cerebrovascular event, or stroke, can be classified as hemorrhagic or ischemic. Hemorrhagic stroke occurs when a weakened blood vessel ruptures and bleeds into the surrounding brain tissue. Ischemic stroke occurs when an obstruction within a blood vessel blocks blood flow to the brain. Most (~80%) cerebrovascular events in high-income countries are ischemic in nature and associated with atherosclerosis as an underlying condition (55, 56).
With respect to vitamin C and cerebrovascular disease, a prospective cohort study that followed more than 2,000 residents of a rural Japanese community for 20 years found that the risk of stroke in those with the highest serum concentrations of vitamin C was 29% lower than in those with the lowest serum concentrations of vitamin C (57). Similarly, the EPIC-Norfolk study, a 10-year prospective cohort study in 20,649 adults, found that individuals with plasma vitamin C concentrations in the top quartile (25%) had a 42% lower risk of stroke compared to those in the lowest quartile (≥66 μmol/L vs. <41 μmol/L) (58). In both the Japanese (57) and EPIC-Norfolk (58) populations, blood vitamin C concentrations were highly correlated with fruit and vegetable intake. Therefore, as in many studies of vitamin C intake and chronic disease risk, it is difficult to separate the effects of vitamin C from the effects of other components of fruit and vegetables. For example, potassium — found at high levels in bananas, potatoes, and other fruit and vegetables — is known to be important in blood pressure regulation, and elevated blood pressure is a major risk factor for stroke (see the article on Potassium). A 2013 meta-analysis of 17 prospective cohort studies reported a 19% lower risk of stroke with the highest versus lowest dietary vitamin C intakes and a 38% lower risk with the highest versus lowest circulating vitamin C concentrations (59).
A randomized, double-blind, placebo-controlled trial in more than 14,000 older men participating in the Physicians' Health Study II (PHS II) found that vitamin C supplementation (500 mg/day) for an average of eight years had no significant effect on the incidence of or mortality from any type of stroke (53). Other trials also failed to show any evidence of an effect of vitamin C on the risk of stroke. A meta-analysis of 10 trials that examined antioxidant vitamins, of which five included vitamin C, found no association between any antioxidant vitamin (vitamin C, vitamin E, or β-carotene), administered alone or in combination, and risk of stroke (60).
Cancer
Overall, observational prospective cohort studies have reported no or modest inverse associations between vitamin C intake and the risk of developing a given type of cancer (37, 61-63). Additional detail is provided below for those cancer subtypes with substantial scientific information obtained from prospective cohort studies. Randomized, double-blind, placebo-controlled trials that have tested the effect of vitamin C supplementation (alone or in combination with other antioxidant nutrients) on cancer incidence or mortality have shown no effect (64).
Breast cancer
Two large prospective studies found dietary vitamin C intake to be inversely associated with breast cancer incidence in certain subgroups. In the Nurses' Health Study, premenopausal women with a family history of breast cancer who consumed an average of 205 mg/day of vitamin C from food had a 63% lower risk of breast cancer than those who consumed an average of 70 mg/day (65). In the Swedish Mammography Cohort, overweight women who consumed an average of 110 mg/day of vitamin C had a 39% lower risk of breast cancer compared to overweight women who consumed an average of 31 mg/day (66). More recent prospective cohort studies have reported no association between dietary and/or supplemental vitamin C intake and breast cancer (67-69).
Stomach cancer
A number of observational studies have found increased dietary vitamin C intake to be associated with decreased risk of gastric (stomach) cancer, and laboratory experiments indicate that vitamin C inhibits the formation of carcinogenic N-nitroso compounds in the stomach (70-72). A nested case-control study in the EPIC study found a 45% lower risk of gastric cancer incidence in individuals in the highest (≥51 μmol/L) versus lowest (<29 μmol/L) quartile of plasma vitamin C concentration; no association was observed between dietary vitamin C intake and gastric cancer (73).
Infection with the bacteria, Helicobacter pylori (H. pylori), is known to increase the risk of stomach cancer and is associated with lower vitamin C content of stomach secretions (74, 75). Although two intervention studies failed to show a reduction in stomach cancer incidence with vitamin C supplementation (35), some research suggests that vitamin C supplementation may be a useful addition to standard H. pylori eradication therapy in reducing the risk of gastric cancer (76). Because vitamin C can inactivate urease (an enzyme that facilitates H. pylori survival and colonization of the gastric mucosa at low pH) in vitro, vitamin C may be most effective as a prophylactic agent in those without achlorhydria (77, 78).
Colon cancer
By pooling data from 13 prospective cohort studies comprising 676,141 participants, it was determined that dietary intake of vitamin C was not associated with colon cancer, while total intake of vitamin C (i.e., from food and supplements) was associated with a 19% reduced risk of colon cancer (79). Each of the cohort studies used self-administered food frequency questionnaires at baseline to assess vitamin C intake. Although the analysis adjusted for several lifestyle and known risk factors, the authors noted that other healthy behaviors and/or folate intake may have confounded the association.
Non-Hodgkin lymphoma
A population-based, prospective study, the Iowa Women's Health Study, collected baseline data on diet and supplement use in 35,159 women (aged 55-69 years) and evaluated the risk of developing non-Hodgkin lymphoma (NHL) over 19 years of follow-up (80). Overall, an inverse association between fruit and vegetable intake and risk of NHL was observed. Additionally, dietary, but not supplemental, intake of vitamin C and other antioxidant nutrients (carotenoids, proanthocyanidins, and manganese) was inversely associated with NHL risk. Another large, multi-center, prospective study — the Women's Health Initiative — that followed 154,363 postmenopausal women for 11 years found that dietary and supplemental vitamin C intake at baseline was inversely associated with diffuse B-cell lymphoma, a subtype of NHL (81).
Other site-specific cancer types
The Physicians' Health Study II was a randomized, placebo-controlled trial that examined the effect of vitamin E (400 IU/day), vitamin C (500 mg/day), and a multivitamin supplement on the risk of cancer in 14,641 middle-aged male physicians over 10.3 years (7.6 years of active treatment plus 2.8 years post-treatment follow-up) (82). Supplementation with vitamin C had no effect on the overall risk of cancer or on the risk of prostate, bladder, or pancreatic cancer; there was a marginal reduction in colorectal cancer incidence with vitamin C compared to placebo (82).
Type 2 diabetes mellitus
In the National Institutes of Health (NIH)-American Association of Retired Persons (AARP) Diet and Health study that included 232,007 participants, the use of vitamin C supplements for at least seven times a week was associated with a 9% lower risk of developing type 2 diabetes mellitus compared to non-supplement use (83). In a cohort of 21,831 adults followed for 12 years in the EPIC-Norfolk study, high plasma vitamin C was found to be strongly associated with a reduced risk of diabetes (84). Additionally, several cross-sectional studies reported inverse associations between circulating vitamin C concentrations and markers of insulin resistance or glucose intolerance, such as glycated hemoglobin (HbA1c) concentration (50, 85, 86). Yet, short-term randomized controlled studies have found no effect of vitamin C supplementation on fasting glucose, fasting insulin, and HbA1c concentrations in healthy individuals (87). It is not known whether supplemental vitamin C could improve markers of glycemic control in subjects at risk of diabetes.
Adverse pregnancy outcomes
A 2015 meta-analysis of 29 randomized controlled trials found that administration of vitamin C during pregnancy, alone or in combination with a few other supplements, failed to reduce the risks of stillbirth, perinatal death, intrauterine growth restriction, preterm birth, premature rupture of membranes, and preeclampsia (88). Nonetheless, vitamin C supplementation led to a 36% lower risk of placental abruption and to a significant increase in gestational age at birth (88). Another meta-analysis of 40 randomized controlled trials in 276,820 women found no effect of vitamin C, alone or combined with vitamin E or multivitamins, when supplemented during pregnancy (starting prior to 20 weeks' gestation), on the risks of overall fetal loss, miscarriage, stillbirth, and congenital malformation (89).
Cigarette smoking during pregnancy causes intrauterine growth restriction and preterm birth, among other pregnancy complications (90, 91), and is the primary cause of childhood respiratory illness (92). For some still unclear reasons, smoking has been associated with a lower risk of preeclampsia during pregnancy (93). A secondary analysis of a multicenter, randomized, double-blind, placebo-controlled trial in nearly 10,000 pregnant women found no reduction in the risk of preeclampsia with supplemental vitamin C (1,000 mg/day) and vitamin E (400 IU/day), regardless of women's smoking status during pregnancy. However, antioxidant supplementation resulted in reduced risks of placental abruption and preterm birth in women who smoked during pregnancy but not in non-smokers (94). Another pilot multicenter trial found better lung function during the first week of life and lower risk of wheezing through one year of age in infants whose smoking mothers were randomized to receive vitamin C (500 mg/day) rather than a placebo during pregnancy (95). The Vitamin C to Decrease the Effects of Smoking in Pregnancy on Infant Lung Function [VCSIP] study is an ongoing trial designed to confirm these preliminary observations using more accurate measurements of pulmonary function in a larger sample of women randomized to receive supplemental vitamin C or placebo (96).
Alzheimer's disease
In the US, Alzheimer's disease (AD) is the most common form of dementia, affecting 5.5 million individuals 65 years and over (97). Oxidative stress, neuroinflammation, β-amyloid plaque deposition, Tau protein-forming tangles, and neuronal cell death in the brain of subjects affected by AD have been associated with cognitive decline and memory loss. Lower vitamin C concentrations in the cerebrospinal fluid (CSF) and brain extracellular matrix of a mouse model of AD were found to increase oxidative stress and accelerate amyloid deposition and disease progression (98). In another AD mouse model that was lacking the ability to synthesize vitamin C, supplementation with a high versus low dose of vitamin C reduced amyloid deposition in the cortex and hippocampus and limited blood-brain barrier impairments and mitochondrial dysfunction (99).
The majority of large, population-based studies examining the relationship of vitamin C intake or supplementation with AD incidence have reported null results (100). In contrast, observational studies reported lower plasma vitamin C concentrations in AD patients compared to cognitively healthy subjects (101) and found better cognitive function or lower risk of cognitive impairment with higher plasma vitamin C (100).
Few studies have measured vitamin C concentration in the CSF, which more closely reflects the vitamin C status of the brain. Vitamin C is concentrated in the brain through a combination of active transport into brain tissue and retention via the blood-brain barrier (100). Although CSF vitamin C is maintained at concentrations several-fold higher than plasma vitamin C, the precise function of vitamin C in cognitive function and AD etiology is not yet fully understood (102). In a small, longitudinal biomarker study in 32 individuals with probable AD, a higher CSF-to-plasma vitamin C ratio at baseline was associated with a slower rate of cognitive decline at one year of follow-up (103). Impaired blood-brain barrier integrity may affect the brain's ability to retain vitamin C and thus to maintain a high CSF-to-plasma vitamin C ratio. The significance of the CSF-to-plasma vitamin C ratio in AD progression requires further study.
The effect of vitamin C supplementation, in combination with other antioxidants, on CSF biomarkers and cognitive function has been examined in only a few trials involving AD patients. In a small (n=23), open-label trial, combined supplementation with vitamin C (1,000 mg/day) and vitamin E (400 IU/day) to AD patients taking a cholinesterase inhibitor significantly increased antioxidant levels and decreased lipoprotein oxidation in CSF after one year, but had no effect on the clinical course of AD compared to controls (104). A similar finding was obtained in a double-blind, randomized controlled trial in which combined supplementation with vitamin C (500 mg/day), vitamin E (800 IU/day), and α-lipoic acid (900 mg/day) for 16 weeks reduced lipoprotein oxidation in CSF but elicited no clinical benefit in individuals with mild-to-moderate AD (n=78) (105). In this latter trial, a greater decline in the Mini Mental State Examination (MMSE) score was observed in the supplemented group, however, the significance of this observation remains unclear. A third placebo-controlled trial in mildly cognitively impaired older adults (ages, 60-75 years) found that one-year supplementation with vitamin C (400 mg/day) and vitamin E (300 mg/day) improved antioxidant blood capacity but had no effect on MMSE scores (106).
At this time, avoidance of vitamin C deficiency or insufficiency, rather than supplementation in replete individuals, seems prudent for the promotion of healthy brain aging (101).
Cataracts
The lens of the eye focuses light, producing a clear, sharp image on the retina, a layer of tissue on the inside back wall of the eyeball. Age-related changes to the lens (thickening, loss of flexibility) and oxidative damage contribute to the formation of cataract, i.e., cloudiness or opacity in the lens that interferes with the clear focusing of images on the retina.
In humans, vitamin C concentration is about 15 to 20 times higher in the aqueous humor — fluid that fills the anterior and posterior chambers of the eye — than in plasma, suggesting that the vitamin may be playing an important role in the eye (107). Decreased vitamin C concentrations in the lens of the eye have been associated with increased severity of cataracts (108). A meta-analysis of observational studies found that a reduced risk of age-related cataract with higher dietary vitamin C intakes in case-control studies and with higher circulating vitamin C concentrations in cross-sectional studies. However, no such associations were found in pooled analyses of prospective cohort studies (109). In fact, two prospective cohort studies in Swedish men (110) and women (111) reported that high-dose single nutrient supplements of vitamin C were associated with an increased risk of cataract, especially in those on corticosteroid therapy.
A 2012 review of nine randomized controlled trials found no substantial effect of β-carotene, vitamin C, and vitamin E, administered individually or in combination over 2.1 to 12 years, on the risk of cataracts or cataract surgery (112). Although trials do not currently support the use of high-dose supplementation with vitamin C in cataract prevention, there is a consistent inverse association observed between high daily intake of fruit and/or vegetables (>5 servings/day) and risk of cataract (113).
Gout
Gout, a condition that afflicts more than 4% of US adults (114), is characterized by abnormally high blood concentrations of uric acid (urate) (115). Urate crystals may form in joints, resulting in inflammation and pain, as well as in the kidneys and urinary tract, resulting in kidney stones. The tendency to exhibit elevated blood uric acid concentrations and develop gout is often inherited; however, dietary and lifestyle modification may be helpful in both the prevention and treatment of gout (116). In an observational study that included 1,387 men, higher intakes of vitamin C were associated with lower serum concentrations of uric acid (117). In a cross-sectional study conducted in 4,576 African Americans, the odds of having hyperuricemia was associated with dietary intakes high in fructose, low in vitamin C, or with high fructose-to-vitamin C ratios (118). A prospective study that followed a cohort of 46,994 men for 20 years found that total daily vitamin C intake was inversely associated with incidence of gout, with higher intakes being associated with greater risk reductions (119). The results of this study also indicated that supplemental vitamin C may be helpful in the prevention of gout (119).
A 2011 meta-analysis of 13 randomized controlled trials in healthy individuals with elevated serum uric acid revealed that vitamin C supplementation (a median dose of 500 mg/day for a median duration of 30 days) modestly reduced serum uric acid concentrations by 0.35 mg/dL compared to placebo (120). Such a reduction falls within the range of assay variability and is unlikely to be clinically significant (121). An eight-week, open-label, controlled trial randomized 40 subjects with gout to receive either allopurinol (standard-of-care), vitamin C, or both treatments (122). The effect of vitamin C, alone or with allopurinol, decreasing serum uric acid was modest and much less than that of allopurinol alone. The trial did not examine the effect of vitamin C on other outcomes associated with gout (122).
Although observational studies suggested that supplemental vitamin C may be helpful to prevent incident and recurrent gout, this has not been demonstrated by intervention studies undertaken thus far. In addition, there is currently little evidence to support a role for vitamin C in the management of patients with gout (123).
Mortality
Two large prospective cohort studies assessed the relationship between dietary and supplemental vitamin C intakes and mortality. In the Vitamins and Lifestyle Study, 55,543 men and women (ages 50-76 years) were questioned at baseline on their use of dietary supplements during the previous 10 years (124). After five years of follow-up, vitamin C supplement use was associated with a small decreased risk of total mortality, although no association was found with cardiovascular disease- or cancer-specific mortality. In the second prospective cohort study, the Diet, Cancer and Health Study, 55,543 Danish adults (ages 50-64 years) were questioned at baseline about their lifestyle, diet, and supplement use during the previous 12 months (125). No association between dietary or supplemental intake of vitamin C and mortality was found after approximately 14 years of follow-up. In contrast, a 2014 meta-analysis of 10 prospective cohort studies in 17,696 women with breast cancer found a lower risk of total and breast cancer-specific mortality with higher supplemental and dietary vitamin C intakes (126). A 2012 meta-analysis of 29 trials found no effect of oral vitamin C, given alone or in combination with other antioxidants, on all-cause mortality (127).
In parallel to these dietary assessment studies, a strong inverse association between plasma vitamin C and mortality from all-causes, cardiovascular disease, and ischemic heart disease (and cancer in men only) was observed in the EPIC-Norfolk multicenter, prospective cohort study (128). After approximately four years of follow-up in 19,496 men and women (ages 45-79 years), a dose-response relationship was observed such that each 20 μmol/L increase in plasma vitamin C was associated with an estimated 20% risk reduction in all-cause mortality. Similarly, higher serum vitamin C concentrations were associated with decreased risks of cancer-specific and all-cause mortality in 16,008 adults from the US National Health and Nutrition Examination Survey (NHANES) III (1994-1998) (129).
Disease Treatment
Cardiovascular disease
Complications of cardiac procedures and surgeries
Periprocedural myocardial injury: Coronary angioplasty (also called percutaneous transluminal coronary angioplasty) is a nonsurgical procedure for treating obstructive coronary heart disease (CHD), including unstable angina pectoris, acute myocardial infarction, and multivessel CHD. Angioplasty involves temporarily inserting and inflating a tiny balloon into the clogged artery to help restore the blood flow to the heart. Periprocedural myocardial injury that occurs in up to one-third of patients undergoing otherwise uncomplicated angioplasty increases the risk of morbidity and mortality at follow-up.
One randomized, placebo-controlled trial has examined the effect of intravenous vitamin C administered to patients with stable angina undergoing elective coronary angioplasty (130). Administration of a 1-gram (g) vitamin C infusion one hour prior to the angioplasty reduced the concentrations of oxidative stress markers and improved microcirculatory perfusion compared to placebo (130). Another trial randomized 532 patients to receive a 3-g vitamin C infusion or a placebo (saline solution) within six hours prior to coronary angioplasty (131). Vitamin C treatment substantially reduced the incidence of periprocedural myocardial injury, as assessed by a reduction in the concentrations of two markers of myocardial injury, namely creatine kinase and troponin-I (131). A recent randomized controlled trial assessed the effect of vitamin C and vitamin E administration on reperfusion damage in patients who experienced acute myocardial infarction and underwent coronary angioplasty (see below) (132).
Myocardial reperfusion injury: Reperfusion injury refers to tissue damage occurring at the time of blood flow restoration (reperfusion) following transient ischemia. The heart muscle may become oxygen-deprived (ischemic) as the result of myocardial infarction or with aortic clamping during coronary artery bypass graft (CABG) surgery. Increased generation of reactive oxygen species (ROS) when the heart muscle's oxygen supply is restored might be an important contributor to myocardial damage occurring at reperfusion (133). Myocardial reperfusion injury leads to complications, such as reperfusion arrhythmias (see Atrial fibrillation) and myocardial stunning.
Vitamin C is depleted during and following cardiac surgery (134) and this might be due to the direct quenching of ROS, the regeneration of other antioxidants, and/or a massive synthesis of catecholamines (dopamine, epinephrine, norepinephrine) (135). Two randomized controlled trials conducted in the 1990s reported a reduction in reperfusion-induced oxidative stress and myocardial injury with intravenous (136) or oral (137) vitamin C administration prior to CABG surgery (reviewed in 135). A more recent randomized, double-blind, placebo-controlled trial has been designed to examine the effect of vitamin C and vitamin E administration on ischemia-reperfusion damage in 99 patients with acute myocardial infarction undergoing coronary angioplasty (132). Vitamin C infusion (sodium ascorbate: 3.20 mmol/min for 1 hour then 0.96 mmol/min for 2 hours) prior to reperfusion followed by oral supplementation with vitamin C (1 g/day) and vitamin E (400 IU/day) for 84 days effectively prevented a reduction in antioxidant capacity at reperfusion and for the next six to eight hours. The protocol also limited microvascular dysfunction (i.e., improved microcirculatory perfusion) and improved left ventricular ejection fraction at discharge (on day 84) (138, 139). However, no difference in the infarct size between antioxidant vitamin treatment and placebo was seen (138).
Atrial fibrillation: Atrial fibrillation is the most common type of cardiac arrhythmia. It is also a common post-cardiac surgery complication, leading to an increased risk of cardiovascular morbidity (e.g., heart failure, stroke) and mortality. Three meta-analyses of prospective cohort studies and randomized controlled trials have reported an overall reduction in the risk of post-operative atrial fibrillation following administration of primarily oral vitamin C (140-142). In most trials, participants received 2 g of vitamin C prior to undergoing CABG or valve replacement surgery and 1 to 2 g/day for five days post-surgery. Although only a minority of trials delivered vitamin C intravenously, this administration route appeared to be more effective at reducing the risk of atrial fibrillation — presumably due to higher plasma concentrations achieved (140). Of note, a subgroup analysis in one of the meta-analyses showed a reduction of post-operative atrial fibrillation with vitamin C in non US-based trials (10 trials) but no effect of vitamin C in US-based trials (5 trials) (140).
Cerebral ischemia-reperfusion injury
A small randomized controlled trial performed in 60 ischemic stroke patients showed that intravenous vitamin C administration (500 mg/day for 10 days, initiated day 1 post-stroke) had no effect on serum markers of oxidative stress or neurological outcomes compared to placebo (143).
Vascular complications of diabetes mellitus
Cardiovascular disease (CVD) is the leading cause of death in individuals with diabetes mellitus. The role of increased oxidative stress in the occurrence of vascular complications in subjects with diabetes has led to hypothesis that higher intakes of antioxidant nutrients could help lower the risk of CVD in diabetic subjects (144). A 2018 meta-analysis of randomized controlled trials investigating the effect of antioxidant vitamin supplementation in patients with type 2 diabetes found that most improvement in markers of oxidative stress and blood glucose control could be attributed to vitamin E (145). Another meta-analysis of trials found no effect of vitamins E and C, alone or in combination, on measures of β-cell function and insulin resistance (146). Yet, most studies were small and of short duration and thus did not assess the consequence of long-term use of antioxidant vitamins on the risk of vascular complications in diabetic patients. One 12-month randomized placebo-controlled trial in 456 participants with type 2 diabetes treated with metformin examined the effect of vitamin C (500 mg/day) or acetylsalicylic acid (aspirin; 100 mg/day) on risk factors for diabetes-related complications such as CVD (147). Both vitamin C and aspirin reduced fasting blood glucose and HbA1c concentrations and improved blood lipid profile in metformin-treated patients. Compared to placebo, both treatments were found to be more likely to limit risk factors contributing to diabetes-related complications, as well as to lower the risk of future cardiovascular events over a 10-year period (estimated using the Framingham risk score) (147).
Of note, it is possible that genetic differences among diabetic patients influence the effect of vitamin C supplementation on cardiovascular risk. In particular, a specific allele of the haptoglobin gene (Hp), namely Hp2, appears to be associated with an increased risk of diabetic vascular complications. Carriers of two copies of the Hp2 allele (Hp2-2) express a Hp protein that has a lower capacity to bind and remove pro-oxidant, free hemoglobin (Hb) from plasma, compared to Hp proteins coded by the Hp1-1 and Hp1-2 genotypes. When the results of the Women's Antioxidant Vitamin Estrogen (WAVE) trial were reanalyzed based on Hp genotype, antioxidant therapy (1,000 mg/day of vitamin C + 800 IU/day of vitamin E) was associated with improvement of coronary atherosclerosis in diabetic women with Hp1-1 genotype but worsening of coronary atherosclerosis in those carrying the Hp2-2 genotype (148). Results from another study by the same investigators suggested that vitamin C could not prevent the oxidation of high-density lipoprotein (HDL)-cholesterol by glycated Hb-Hp2-2 complexes in vitro nor restore impaired HDL function in diabetic mice carrying the Hp2-2 genotype (149).
Sepsis
Sepsis and septic shock — defined as persistent sepsis-induced low blood pressure — are associated with elevated mortality rates in critically ill patients (150, 151). Because systemic inflammatory responses involve excessive oxidative stress, it has been suggested that providing antioxidant nutrients like vitamin C may improve the outcome of critically ill patients in intensive care units. In addition, hypovitaminosis C is common in critically ill patients, especially in those with septic shock, and persists despite enteral/parenteral nutritional therapy providing recommended amounts of vitamin C (152). Vitamin C requirements are likely to be increased in this population due to the hypermetabolic response driven by the systemic inflammatory reaction (152, 153). Intravenous administration of 50 mg or 200 mg of vitamin C per kg per day for 96 hours to patients with sepsis admitted in intensive care unit was found to correct vitamin C deficiency. Vitamin C also prevented the rise of Sequential Organ Failure Assessment (SOFA) and Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) II scores — used to assess severity of illness and risk of mortality — observed in placebo-treated patients (154). Vitamin C infusion also lowered the concentration of markers of inflammation and endothelial injury in patients compared to placebo (154). In another randomized, double-blind, controlled trial in 28 critically ill patients with septic shock, infusion of 25 mg of vitamin C per kg every six hours for 72 hours significantly limited the requirement to vasopressor norepinephrine — decreasing both the dose and duration of treatment — and dramatically improved the 28-day survival rate (155). Similar results have been reported in septic patients given intravenous vitamin C (1.5 g/6 h), hydrocortisone (50 mg/6 h), and thiamin (200 mg/12 h) until hospital discharge. Compared to standard-of-care, this intervention cocktail more than halved the mean duration of vasopressor use (18.3 h versus 54.9 h) and reduced the odds of mortality by nearly 90% (156). Although intravenous vitamin C administration appears to be safe and well tolerated, there is a non-negligible risk of oxalate nephropathy (a rare cause of kidney failure) in these critically ill patients (157).
See also the section on Sepsis in the Symposium on Vitamin C — Part of the LPI's 9th International Conference on Diet and Optimum Health.
Cancer
Route of administration
Studies in the 1970s and 1980s conducted by Linus Pauling, Ewan Cameron, and colleagues suggested that large doses of vitamin C (10 g/day infused intravenously for 10 days followed by at least 10 g/day orally indefinitely) were helpful in increasing the survival time and improving the quality of life of terminal cancer patients (158). Controversy surrounding the efficacy of vitamin C in cancer treatment ensued, leading to the recognition that the route of vitamin C administration is critical (22, 159). Compared to orally administered vitamin C, intravenous vitamin C can result in 30 to 70-fold higher plasma vitamin C concentrations (25). Higher plasma concentrations achieved via intravenous vitamin C administration are comparable to those that are toxic to cancer cells in culture. The anticancer mechanism of intravenous vitamin C action is under investigation. It may involve the production of high levels of hydrogen peroxide, selectively toxic to cancer cells (22, 160-162), or the deactivation of hypoxia inducible factor, a prosurvival transcription factor that protects cancer cells from various forms of stress (159, 163, 164). Vitamin C likely also plays a role in the maintenance of genome integrity and in the protection against cellular transformation through regulating DNA and histone demethylating enzymes (see Function) (165).
Safety
Current evidence from controlled clinical trials indicates that intravenous vitamin C is generally safe and well tolerated in cancer patients. Of note, because intravenous administration of 80 g of vitamin C precipitated hemolytic anemia in two subjects with glucose-6-phosphate dehydrogenase deficiency, patients due to receive high-dose vitamin C infusion are systematically screened for this genetic disorder (166). Four phase I clinical trials in patients with advanced cancer found that intravenous administration of vitamin C at doses up to 1.5 g/kg of body weight (equivalent to about 100 g/day for an average weight [70 kg] person) and 70 to 80 g/m2 was well tolerated and safe in pre-screened patients (167-170). A few observational studies in cancer patients undergoing chemotherapy and/or radiotherapy reported that complementary intravenous vitamin C treatment was associated with a reduction in treatment-associated side effects and an improved quality of life (171). A phase I study in nine patients with metastatic pancreatic cancer showed that millimolar concentrations of plasma vitamin C could be reached safely when administered in conjunction with the cancer chemotherapy drugs, gemcitabine and erlotinib (168).
Sensitivity to vitamin C
Retrospective in vitro colony formation assays revealed that patient leukemic cells displayed variable sensitivity to vitamin C treatment: leukemic cells from seven out of the nine patients who experienced a significant clinical benefit were sensitive to vitamin C in vitro (i.e., "responders"); the leukemic cells from the remaining six patients were not sensitive to vitamin C (i.e., "non-responders"). Thus, in vitro vitamin C sensitivity assays may provide predictive value for the clinical response to intravenous vitamin C treatment. The mechanisms underlying differential sensitivity to vitamin C are under investigation. In vitro experiments performed using 11 different cancer cell lines demonstrated that sensitivity to vitamin C correlated with the expression of catalase, an enzyme involved in the decomposition of hydrogen peroxide (172). Approximately one-half of the cell lines tested were resistant to vitamin C cytotoxicity, a response associated with high levels of catalase activity.
Sensitivity to vitamin C may also be determined by the expression of sodium-dependent vitamin C transporter-2 (SVCT-2), which transports vitamin C into cells (173). Higher SVCT-2 levels were associated with enhanced sensitivity to vitamin C in nine different breast cancer cell lines. Moreover, SVCT-2 was significantly expressed in 20 breast cancer tissue samples, but weakly expressed in normal tissues. Finally, mutations in genes coding for vitamin C-dependent TET demethylases, mutations that are common in cancer cells, may also contribute to resistance to vitamin C treatment (165).
Efficacy
Current evidence of the efficacy of intravenous vitamin C in cancer patients is limited to observational studies, uncontrolled interventions, and case reports (174, 175). There is a need for larger, longer-duration phase II clinical trials that test the efficacy of intravenous vitamin C in disease progression and overall survival (176).
See also the section on Cancer in the Symposium on Vitamin C — Part of the LPI's 9th International Conference on Diet and Optimum Health.
Common cold
The work of Linus Pauling stimulated public interest in the use of doses greater than 1 g/day of vitamin C to prevent the common cold (177). In the past 40 years, numerous placebo-controlled trials have examined the effect of vitamin C supplementation on the prevention and treatment of colds. A 2013 meta-analysis of 53 placebo-controlled trials evaluated the effect of vitamin C supplementation on the incidence, duration, or severity of the common cold when taken as a continuous daily supplement (43 trials) or as therapy upon onset of cold symptoms (10 trials) (178). Regarding the incidence of colds, a difference was observed between two groups of participants. Regular supplementation with vitamin C (0.25 to 2 g/day) did not reduce the incidence of colds in the general population (23 trials); however, in participants undergoing heavy physical stress (e.g., marathon runners, skiers, or soldiers in subarctic conditions), vitamin C supplementation halved the incidence of colds (5 trials). A benefit of regular vitamin C supplementation was also seen in the duration of colds, with a greater benefit in children than in adults: The pooled effect of vitamin C supplementation was a 14% reduction in cold duration in children and an 8% reduction in adults. Finally, no significant effect of vitamin C supplementation (1-8 g/day) was observed in therapeutic trials in which vitamin C was administered after cold symptoms occurred.
In addition, a 2013 systematic review by the same investigators identified only two small randomized, double-blind, placebo-controlled trials that examined the effect of vitamin C on the incidence of respiratory infection-induced asthma (179). One trial found that vitamin C supplementation (1 g/day) for 14 weeks reduced the risk of asthma attacks precipitated by respiratory infection. The other trial randomized subjects diagnosed with infection-related asthma to receive 5 g/day of vitamin C or a placebo for one week; a lower proportion of participants was found to present with bronchial hypersensitivity to histamine — which characterizes chronic asthma — in the vitamin C group compared to the control group (reviewed in 179). These observations need to be confirmed in larger, well-designed trials.
Asthma
A 2013 systematic review identified 11 randomized controlled studies that evaluated the effect of vitamin C on asthma (eight trials) or exercise-induced bronchoconstriction (three trials) (180). Exercise-induced bronchoconstriction is a transient narrowing of the airways that occurs after exercise and is indicated by a ≥10% decline in Forced Expiratory Volume in 1 second (FEV1). In the three trials that included a total of 40 participants with exercise-induced bronchoconstriction, vitamin C administration before exercise (a 0.5-g dose on two subsequent days in one trial, a single dose of 2 g in the second trial, and 1.5 g daily for two weeks in the third trial) significantly reduced the exercise-induced decline in FEV1. Among the five out of eight trials in asthmatic subjects that reported on FEV1 outcomes, none found a difference between vitamin C supplementation and placebo (180).
Lead toxicity
Although the use of lead paint and leaded gasoline has been discontinued in the US, lead toxicity continues to be a significant health problem, especially in children living in urban areas. Abnormal growth and development have been observed in infants of women exposed to lead during pregnancy, while children who are chronically exposed to lead are more likely to develop learning disabilities, behavioral problems, and to have a low IQ. In adults, lead toxicity may result in kidney damage, high blood pressure, and anemia.
Several cross-sectional studies have reported an inverse association between vitamin C status and blood lead concentration. For instance, in a study of 747 older men, blood lead concentration was significantly higher in those who reported total dietary vitamin C intakes averaging less than 109 mg/day compared to those with higher vitamin C intakes (181). A much larger study of 19,578 people, including 4,214 children from 6 to 16 years of age, found higher serum vitamin C concentrations to be associated with significantly lower blood lead concentrations (182). A US national survey of more than 10,000 adults found that blood lead concentrations were inversely related to serum vitamin C concentrations (183).
Cigarette smoking or second-hand exposure to cigarette smoke contributes to increased blood lead concentration and a state of chronic low-level lead exposure. An intervention trial in 75 adult male smokers found that supplementation with 1,000 mg/day of vitamin C resulted in significantly lower blood lead concentration over a four-week treatment period compared to placebo (184). A lower dose of 200 mg/day did not significantly affect blood lead concentration, although serum vitamin C concentrations were not different from those in the group who took 1,000 mg/day.
The mechanism(s) by which vitamin C reduces blood lead concentration is not known, yet it has been proposed that vitamin C could inhibit intestinal absorption (184) or enhance urinary excretion of lead (185).
Sources
Unlike plants and most animals, humans have lost the ability to synthesize vitamin C endogenously and therefore have an essential dietary requirement for this vitamin (see The Recommended Dietary Allowance). Results from 7,277 participants in the US National Health and Nutrition Examination Survey (NHANES) 2003-2004 indicated that an estimated 7.1% of individuals ages ≥6 years were deficient in vitamin C — based on serum vitamin C concentrations <11.4 μmol/L (36). The national study identified smokers and those of lower socioeconomic status to both be at higher risk for vitamin C deficiency (36).
Food sources
As shown in Table 3, different fruit and vegetables vary in their vitamin C content, but five servings (2½ cup-equivalents) of a variety of fruit and vegetables should average out to about 150 to 200 mg of vitamin C, especially if vitamin C-rich fruits are consumed. If you wish to check foods for their vitamin C content, search USDA's FoodData Central.
| Food | Serving | Vitamin C (mg) |
|---|---|---|
| Kiwifruit, Zespri SunGold | 1 fruit (81 g) | 131 |
| Grapefruit juice, pink, raw | ¾ cup (6 ounces) | 94 |
| Orange juice, raw | ¾ cup (6 ounces) | 93 |
| Strawberries | 1 cup, whole | 85 |
| Grapefruit juice, white, raw | ¾ cup (6 ounces) | 70 |
| Kiwifruit | 1 fruit (74 g) | 69 |
| Orange | 1 medium | 65 |
| Sweet red pepper, raw | ½ cup, chopped | 59 |
| Broccoli, cooked | ½ cup | 51 |
| Grapefruit, raw | ½ medium | 44 |
| Brussel sprouts, cooked | ½ cup | 37 |
| Potato, white, flesh and skin | 1 medium, baked | 22 |
| Tomato, red, ripe, raw | 1 medium | 17 |
| Banana, raw | 1 medium | 10 |
| Apple, raw | 1 medium | 8 |
| Spinach, raw | 1 cup | 8 |
Supplements
Vitamin C (L-ascorbic acid) is available in many forms, but there is little scientific evidence that any one form is better absorbed or more effective than another. Most experimental and clinical research uses ascorbic acid or its sodium salt, called sodium ascorbate. Natural and synthetic L-ascorbic acid are chemically identical and there are no known differences regarding biological activities or bioavailability (186).
Mineral ascorbates
Mineral salts of vitamin C are considered less acidic than vitamin C and therefore are considered "buffered." Some people find them less irritating to the gastrointestinal tract than ascorbic acid. Sodium ascorbate and calcium ascorbate are the most common forms, although a number of other mineral ascorbates are available. Sodium ascorbate provides 111 mg of sodium (889 mg of ascorbic acid) per 1,000 mg of sodium ascorbate, and calcium ascorbate generally provides 90 to 110 mg of calcium (890-910 mg of ascorbic acid) per 1,000 mg of calcium ascorbate.
Vitamin C with flavonoids
Flavonoids are a class of water-soluble plant pigments that are often found in vitamin C-rich fruit and vegetables, especially citrus fruit and berries (see the article on Flavonoids). There is little evidence that the flavonoids in most commercial preparations increase the bioavailability or efficacy of vitamin C (187). Some, yet not all, studies in animal models such as vitamin C-deficient guinea pigs or genetically scorbutic rats found an increased uptake of vitamin C in peripheral circulation and specific organs in the presence of flavonoids. However, studies conducted in humans found no differences in bioavailability of vitamin C from flavonoid-rich whole fruit or fruit juice and synthetic vitamin C (reviewed in 186).
Vitamin C and metabolites
One supplement, Ester-C®, contains mainly calcium ascorbate and includes small amounts of the vitamin C metabolites, dehydroascorbic acid (oxidized ascorbic acid), calcium threonate, and trace amounts of xylonate and lyxonate. Although these metabolites are purported to increase the bioavailability of vitamin C, the only published study in humans addressing this issue found no difference between Ester-C® and commercially available vitamin C tablets with respect to the absorption and urinary excretion of vitamin C (187). Ester-C® should not be confused with ascorbyl palmitate, which is also marketed as "vitamin C ester" (see below).
Ascorbyl palmitate
Ascorbyl palmitate is a vitamin C ester (i.e., ascorbic acid linked to a fatty acid). In this case, vitamin C is esterified to the saturated fatty acid, palmitic acid, resulting in a fat-soluble form of vitamin C. Ascorbyl palmitate has been added to a number of skin creams due to interest in its antioxidant properties, as well as its importance in collagen synthesis (see the separate article, Vitamin C and Skin Health) (188). Although ascorbyl palmitate is also available as an oral supplement, most of it is likely hydrolyzed to ascorbic acid and palmitic acid in the digestive tract before it is absorbed (189). Ascorbyl palmitate is marketed as "vitamin C ester," which should not be confused with Ester-C® (see above).
Other formulations of vitamin C
One small placebo-controlled, cross-over trial in 11 men showed that the oral administration of 4 g of vitamin C resulted in a greater vitamin C concentration in plasma over a four-hour period when vitamin C was encapsulated in liposomes compared to unencapsulated vitamin C (190). Although liposomal encapsulation could increase vitamin C bioavailability, plasma vitamin C concentrations were much lower than those achieved with intravenous vitamin C administration (190).
For a more detailed review of scientific research on the bioavailability of different forms of vitamin C, see The Bioavailability of Different Forms of Vitamin C.
Safety
Toxicity
A number of possible adverse health effects of very large doses of vitamin C have been identified, mainly based on in vitro experiments or isolated case reports, and include genetic mutations, birth defects, cancer, atherosclerosis, kidney stones, "rebound scurvy," increased oxidative stress, excess iron absorption, vitamin B12 deficiency, and erosion of dental enamel. However, none of these alleged adverse health effects have been confirmed in subsequent studies, and there is no reliable scientific evidence that doses of vitamin C up to 10 g/day in adults are toxic or detrimental to health. The concern of kidney stone formation with vitamin C supplementation is discussed below.
With the latest RDA published in 2000, a tolerable upper intake level (UL) for vitamin C was set for the first time (Table 4). A UL of 2 g (2,000 mg) daily was recommended in order to prevent generally healthy adults from experiencing diarrhea and gastrointestinal disturbances (35). Such symptoms are not generally serious, especially if they resolve with temporary discontinuation of vitamin C supplementation.
| Age Group | UL (mg/day) |
|---|---|
| Infants 0-12 months | Not possible to establish* |
| Children 1-3 years | 400 |
| Children 4-8 years | 650 |
| Children 9-13 years | 1,200 |
| Adolescents 14-18 years | 1,800 |
| Adults 19 years and older | 2,000 |
| *Source of intake should be from foods or formula only. | |
Kidney stones
Because oxalate is a metabolite of vitamin C, there is some concern that high vitamin C intake could increase the risk of calcium oxalate kidney stones. Some (24, 191, 192), but not all (193-195), studies have reported that supplemental vitamin C increases urinary oxalate concentrations. Whether any increase in oxalate levels would translate to an elevation in risk for kidney stones has been examined in several epidemiological studies. Two large prospective cohort studies, one following 45,251 men for six years and the other following 85,557 women for 14 years, reported that consumption of ≥1,500 mg of vitamin C daily did not increase the risk of kidney stone formation compared to those consuming <250 mg daily (196, 197). On the other hand, two other large prospective studies reported that a high intake of vitamin C was associated with an increased risk of kidney stone formation in men (198, 199). Specifically, the Health Professionals Follow-Up Study collected data on dietary and supplemental vitamin C intake every four years in 45,619 male health professionals (ages 40-75 years) (198). After 14 years of follow-up, it was found that men who consumed ≥1,000 mg/day of vitamin C had a 41% higher risk of kidney stones compared to men consuming <90 mg of vitamin C daily. In the Cohort of Swedish Men study, self-reported use of single-nutrient vitamin C supplements (taken seven or more times per week) at baseline was associated with a two-fold higher risk of incident kidney stones among 48,840 men (ages 45-79 years) followed for 11 years (199). Despite conflicting results, it may be prudent for individuals predisposed to oxalate kidney stone formation to avoid high-dose vitamin C supplementation.
Drug interactions
Overall, evidence suggesting specific drugs can lower blood vitamin C concentrations in humans is limited. Dihydropyridine calcium channel blockers (e.g., nicardipine, nifedipine) can inhibit vitamin C uptake by intestinal cells in vitro. However, a reduction in blood vitamin C concentrations with these drugs has not been reported in humans (200). Aspirin can impair vitamin C status if taken frequently (201).
Conversely, there are case reports suggesting that supplemental vitamin C may lower blood concentrations of some medications, such as fluphenazine (the antipsychotic drug, Prolixin) and indinavir (the antiretroviral drug, Crixivan) (200). There is some evidence, though controversial, that vitamin C interacts with anticoagulant medications like warfarin (Coumadin). Large doses of vitamin C may block the action of warfarin and thus lower its effectiveness. Individuals on anticoagulants should limit their vitamin C intake to <1 g/day and have their prothrombin time monitored by the clinician following their anticoagulant therapy (200). In addition, vitamin C may bind aluminum in the gut and increase the absorption of aluminum-containing compounds (e.g., aluminum-containing antacids, aluminum-containing phosphate binders). People with impaired kidney function may be at risk for aluminum toxicity when supplemental vitamin C is taken at the same time as these compounds (200, 201). Finally, supplemental vitamin C may increase blood estrogen concentrations in women using oral contraceptives or hormone replacement therapy (200).
The potential effect of antioxidants during chemotherapy is not well understood, yet only likely to be an issue if a specific chemotherapeutic agent acts through an oxidative mechanism, which is uncommon (171). It is not clear whether vitamin C given parenterally could diminish or increase the efficacy of chemotherapy drugs — in particular, akylating agents (e.g., cyclophosphamide, busulfan), antitumor antibiotics (e.g., doxorubicin, bleomycin), and arsenic trioxide. Patients are advised to discuss with their oncologist before using vitamin C supplements (200, 201).
Because high doses of vitamin C have also been found to interfere with the interpretation of certain laboratory tests (e.g., serum bilirubin, serum creatinine, and the stool guaiac assay for occult blood), it is important to inform one's health care provider of any recent supplement use.
Antioxidant supplements and HMG-CoA reductase inhibitors (statins)
A three-year randomized controlled trial in 160 patients with documented coronary heart disease and low blood HDL concentrations found that a combination of simvastatin (Zocor) and niacin increased HDL concentration, inhibited the progression of coronary artery stenosis (narrowing), and decreased the frequency of cardiovascular events, such as myocardial infarction and stroke (202). Surprisingly, when an antioxidant combination (1,000 mg vitamin C, 800 IU vitamin E, 100 mg selenium, and 25 mg β-carotene daily) was taken with the simvastatin-niacin combination, the protective effects were diminished. Since the antioxidants were taken together in this trial, the individual contribution of vitamin C cannot be determined. In contrast, a much larger trial in more than 20,000 men and women with coronary heart disease or diabetes mellitus found that simvastatin and an antioxidant combination (600 mg vitamin E, 250 mg vitamin C, and 20 mg β-carotene daily) did not diminish the cardioprotective effects of simvastatin therapy over a five-year period (203). These contradictory findings indicate that further research is needed on potential interactions between antioxidant supplements and cholesterol-lowering drugs, such as HMG-CoA reductase inhibitors (statins).
Does vitamin C promote oxidative damage under physiological conditions?
Vitamin C is known to function as a highly effective antioxidant in living organisms. However, in test tube experiments, vitamin C can interact with some free metal ions and lead to the generation of potentially damaging free radicals. Although free metal ions are not generally found under physiological conditions, the idea that high doses of vitamin C might be able to promote oxidative damage in vivo has received a great deal of attention. Widespread publicity has been given to a few studies suggesting a pro-oxidant effect of vitamin C (204, 205), but these studies turned out to be either flawed or of no physiological relevance. A comprehensive review of the literature found no credible scientific evidence that supplemental vitamin C promotes oxidative damage under physiological conditions or in humans (206).
Linus Pauling Institute Recommendation
Combined evidence from metabolic, pharmacokinetic, and observational studies, and from randomized controlled trials supports consuming sufficient vitamin C to achieve plasma concentrations of at least 60 μmol/L. While most generally healthy young adults can achieve these plasma concentrations with daily vitamin C intake of at least 200 mg/day, some individuals may have a lower vitamin C absorptive capacity than what is currently documented. Thus, the Linus Pauling Institute recommends a vitamin C intake of 400 mg daily for adults to ensure replete tissue concentrations (29) — an amount substantially higher than the RDA yet with minimal risk of side effects.
This recommendation can be met through food if the diet includes at least several servings of vitamin C-rich fruit and vegetables (e.g., citrus fruit, kiwifruit, peppers; see Food sources) as part of the daily recommended fruit and vegetable intake (see article on Fruit and Vegetables). Most multivitamin supplements provide at least 60 mg of vitamin C.
Older adults (>50 years)
Whether older adults have higher requirements for vitamin C is not yet known with certainty, yet some older populations have been found to have vitamin C intakes considerably below the RDA of 75 and 90 mg/day for women and men, respectively (207). A vitamin C intake of at least 400 mg daily may be particularly important for older adults who are at higher risk for age-related chronic diseases. Pharmacokinetic studies in older adults have not yet been conducted, but there is some evidence suggesting that the efficiency of one of the molecular mechanisms for the cellular uptake of vitamin C declines with age (208). Because maximizing blood concentrations of vitamin C may be important in protecting against oxidative damage to cells and biological molecules, a vitamin C intake of at least 400 mg daily might benefit older adults who are at higher risk for chronic diseases caused, in part, by oxidative damage, such as heart disease, stroke, certain cancers, and cataract.
For more information on the difference between Dr Linus Pauling's recommendation and the Linus Pauling Institute's recommendation for vitamin C intake, select the highlighted text.
Authors and Reviewers
Originally written in 2000 by:
Jane Higdon, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in November 2002 by:
Jane Higdon, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in September 2003 by:
Jane Higdon, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in December 2004 by:
Jane Higdon, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in January 2006 by:
Jane Higdon, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in September 2009 by:
Victoria J. Drake, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in November 2013 by:
Giana Angelo, Ph.D.
Linus Pauling Institute
Oregon State University
Updated in July 2018 by:
Barbara Delage, Ph.D.
Linus Pauling Institute
Oregon State University
Reviewed in December 2018 by:
Anitra C. Carr, Ph.D.
Research Associate Professor
Department of Pathology & Biomedical Science
University of Otago
Christchurch, New Zealand
Reviewed in December 2018 by:
Alexander J. Michels, Ph.D.
Research Associate
Linus Pauling Institute
Oregon State University
Copyright 2000-2021 Linus Pauling Institute
References:
1. Levine M, Padayatty SJ. Vitamin C. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease, 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:416-426.
2. Englard S, Seifter S. The biochemical functions of ascorbic acid. Annu Rev Nutr. 1986;6:365-406. (PubMed)
3 Camarena V, Wang G. The epigenetic role of vitamin C in health and disease. Cell Mol Life Sci. 2016;73(8):1645-1658. (PubMed)
4 Young JI, Zuchner S, Wang G. Regulation of the epigenome by vitamin C. Annu Rev Nutr. 2015;35:545-564. (PubMed)
5 Jariwalla RJ, Harakeh S. Antiviral and immunomodulatory activities of ascorbic acid. In: Harris JR, ed. Subcellular Biochemistry. Vol. 25. Ascorbic Acid: Biochemistry and Biomedical Cell Biology. New York: Plenum Press; 1996:215-231.
6 Kennes B, Dumont I, Brohee D, Hubert C, Neve P. Effect of vitamin C supplements on cell-mediated immunity in old people. Gerontology. 1983;29(5):305-310. (PubMed)
7 Panush RS, Delafuente JC, Katz P, Johnson J. Modulation of certain immunologic responses by vitamin C. III. Potentiation of in vitro and in vivo lymphocyte responses. Int J Vitam Nutr Res Suppl. 1982;23:35-47. (PubMed)
8 Prinz W, Bortz R, Bregin B, Hersch M. The effect of ascorbic acid supplementation on some parameters of the human immunological defence system. Int J Vitam Nutr Res. 1977;47(3):248-257. (PubMed)
9 Vallance S. Relationships between ascorbic acid and serum proteins of the immune system. Br Med J. 1977;2(6084):437-438. (PubMed)
10. Anderson R, Oosthuizen R, Maritz R, Theron A, Van Rensburg AJ. The effects of increasing weekly doses of ascorbate on certain cellular and humoral immune functions in normal volunteers. Am J Clin Nutr. 1980;33(1):71-76. (PubMed)
11. Levy R, Shriker O, Porath A, Riesenberg K, Schlaeffer F. Vitamin C for the treatment of recurrent furunculosis in patients with impaired neutrophil functions. J Infect Dis. 1996;173(6):1502-1505. (PubMed)
12. Bergsten P, Amitai G, Kehrl J, Dhariwal KR, Klein HG, Levine M. Millimolar concentrations of ascorbic acid in purified human mononuclear leukocytes. Depletion and reaccumulation. J Biol Chem. 1990;265(5):2584-2587. (PubMed)
13. Evans RM, Currie L, Campbell A. The distribution of ascorbic acid between various cellular components of blood, in normal individuals, and its relation to the plasma concentration. Br J Nutr. 1982;47(3):473-482. (PubMed)
14. Jariwalla RJ, Harakeh S. Mechanisms underlying the action of vitamin C in viral and immunodeficiency disease. In: Packer L, Fuchs J, eds. Vitamin C in Health and Disease. New York: Macel Dekker, Inc.; 1997:309-322.
15. Alberts B, Bray D, Lewis J, Raff M. Differentiated cells and the maintenance of tissues. Molecular Biology of the Cell. 3rd ed. New York: Garland Publishing, Inc.; 1994:1139-1193.
16. Pauling L. The immune system. How to Live Longer and Feel Better. 20th Anniversary ed. Corvallis: Oregon State University Press; 2006:105-111.
17. Dahl H, Degre M. The effect of ascorbic acid on production of human interferon and the antiviral activity in vitro. Acta Pathol Microbiol Scand B. 1976;84B(5):280-284. (PubMed)
18. Carr AC, Maggini S. Vitamin C and immune function. Nutrients. 2017;9(11). (PubMed)
19. Lykkesfeldt J, Poulsen HE. Is vitamin C supplementation beneficial? Lessons learned from randomised controlled trials. Br J Nutr. 2010;103(9):1251-1259. (PubMed)
20. Michels AJ, Frei B. Myths, artifacts, and fatal flaws: identifying limitations and opportunities in vitamin C research. Nutrients. 2013;5(12):5161-5192. (PubMed)
21. Johnston CS. Vitamin C. In: Erdman JWJ, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Ames, Iowa: Wiley-Blackwell; 2012:248-260.
22. Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2(2):78-88. (PubMed)
23. Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci U S A. 2001;98(17):9842-9846. (PubMed)
24. Levine M, Conry-Cantilena C, Wang Y, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A. 1996;93(8):3704-3709. (PubMed)
25. Padayatty SJ, Sun H, Wang Y, et al. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004;140(7):533-537. (PubMed)
26. Carr AC, Bozonet SM, Pullar JM, Simcock JW, Vissers MC. Human skeletal muscle ascorbate is highly responsive to changes in vitamin C intake and plasma concentrations. Am J Clin Nutr. 2013;97(4):800-807. (PubMed)
27. Michels AJ, Hagen TM, Frei B. Human genetic variation influences vitamin C homeostasis by altering vitamin C transport and antioxidant enzyme function. Annu Rev Nutr. 2013;33:45-70. (PubMed)
28. Carr AC, Pullar JM, Bozonet SM, Vissers MC. Marginal ascorbate status (hypovitaminosis C) results in an attenuated response to vitamin C supplementation. Nutrients. 2016;8(6). (PubMed)
29. Frei B, Birlouez-Aragon I, Lykkesfeldt J. Authors' perspective: What is the optimum intake of vitamin C in humans? Crit Rev Food Sci Nutr. 2012;52(9):815-829. (PubMed)
30. Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281(15):1415-1423. (PubMed)
31. Lykkesfeldt J, Christen S, Wallock LM, Chang HH, Jacob RA, Ames BN. Ascorbate is depleted by smoking and repleted by moderate supplementation: a study in male smokers and nonsmokers with matched dietary antioxidant intakes. Am J Clin Nutr. 2000;71(2):530-536. (PubMed)
32. Sauberlich HE. A history of scurvy and vitamin C. In: Packer L, Fuchs J, eds. Vitamin C in health and disease. New York: Marcel Decker, Inc.; 1997:1-24.
33. Stephen R, Utecht T. Scurvy identified in the emergency department: a case report. J Emerg Med. 2001;21(3):235-237. (PubMed)
34. Weinstein M, Babyn P, Zlotkin S. An orange a day keeps the doctor away: scurvy in the year 2000. Pediatrics. 2001;108(3):E55. (PubMed)
35. Food and Nutrition Board, Institute of Medicine. Vitamin C. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, D.C.: National Academy Press; 2000:95-185. (National Academy Press)
36. Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90(5):1252-1263. (PubMed)
37. Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr. 1999;69(6):1086-1107. (PubMed)
38. Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO, Lerman A. Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta-analysis. J Am Heart Assoc. 2015;4(11). (PubMed)
39. Ashor AW, Lara J, Mathers JC, Siervo M. Effect of vitamin C on endothelial function in health and disease: a systematic review and meta-analysis of randomised controlled trials. Atherosclerosis. 2014;235(1):9-20. (PubMed)
40. Forman JP, Choi H, Curhan GC. Fructose and vitamin C intake do not influence risk for developing hypertension. J Am Soc Nephrol. 2009;20(4):863-871. (PubMed)
41. Block G, Jensen CD, Norkus EP, Hudes M, Crawford PB. Vitamin C in plasma is inversely related to blood pressure and change in blood pressure during the previous year in young Black and White women. Nutr J. 2008;7:35. (PubMed)
42. Moran JP, Cohen L, Greene JM, et al. Plasma ascorbic acid concentrations relate inversely to blood pressure in human subjects. Am J Clin Nutr. 1993;57(2):213-217. (PubMed)
43. Myint PK, Luben RN, Wareham NJ, Khaw KT. Association between plasma vitamin C concentrations and blood pressure in the European prospective investigation into cancer-Norfolk population-based study. Hypertension. 2011;58(3):372-379. (PubMed)
44. Buijsse B, Jacobs DR, Jr., Steffen LM, Kromhout D, Gross MD. Plasma ascorbic acid, a priori diet quality score, and incident hypertension: a prospective cohort study. PLoS One. 2015;10(12):e0144920. (PubMed)
45. Juraschek SP, Guallar E, Appel LJ, Miller ER, 3rd. Effects of vitamin C supplementation on blood pressure: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;95(5):1079-1088. (PubMed)
46 Knekt P, Ritz J, Pereira MA, et al. Antioxidant vitamins and coronary heart disease risk: a pooled analysis of 9 cohorts. Am J Clin Nutr. 2004;80(6):1508-1520. (PubMed)
47. Ye Z, Song H. Antioxidant vitamins intake and the risk of coronary heart disease: meta-analysis of cohort studies. Eur J Cardiovasc Prev Rehabil. 2008;15(1):26-34. (PubMed)
48 Kubota Y, Iso H, Date C, et al. Dietary intakes of antioxidant vitamins and mortality from cardiovascular disease: the Japan Collaborative Cohort Study (JACC) study. Stroke. 2011;42(6):1665-1672. (PubMed)
49. Pfister R, Sharp SJ, Luben R, Wareham NJ, Khaw KT. Plasma vitamin C predicts incident heart failure in men and women in European Prospective Investigation into Cancer and Nutrition-Norfolk prospective study. Am Heart J. 2011;162(2):246-253. (PubMed)
50. Carter P, Gray LJ, Troughton J, Khunti K, Davies MJ. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis. BMJ. 2010;341:c4229. (PubMed)
51. Dehghan M, Akhtar-Danesh N, McMillan CR, Thabane L. Is plasma vitamin C an appropriate biomarker of vitamin C intake? A systematic review and meta-analysis. Nutr J. 2007;6:41. (PubMed)
52. Al-Khudairy L, Flowers N, Wheelhouse R, et al. Vitamin C supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2017;3:Cd011114. (PubMed)
53. Sesso HD, Buring JE, Christen WG, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2008;300(18):2123-2133. (PubMed)
54. Roberts LJ, 2nd, Traber MG, Frei B. Vitamins E and C in the prevention of cardiovascular disease and cancer in men. Free Radic Biol Med. 2009;46(11):1558. (PubMed)
55. Hankey GJ. The global and regional burden of stroke. Lancet Glob Health. 2013;1(5):e239-240. (PubMed)
56. Tsivgoulis G, Safouris A, Kim DE, Alexandrov AV. Recent Advances in Primary and Secondary Prevention of Atherosclerotic Stroke. J Stroke. 2018;20(2):145-166. (PubMed)
57. Yokoyama T, Date C, Kokubo Y, Yoshiike N, Matsumura Y, Tanaka H. Serum vitamin C concentration was inversely associated with subsequent 20-year incidence of stroke in a Japanese rural community. The Shibata study. Stroke. 2000;31(10):2287-2294. (PubMed)
58. Myint PK, Luben RN, Welch AA, Bingham SA, Wareham NJ, Khaw KT. Plasma vitamin C concentrations predict risk of incident stroke over 10 y in 20 649 participants of the European Prospective Investigation into Cancer Norfolk prospective population study. Am J Clin Nutr. 2008;87(1):64-69. (PubMed)
59. Chen GC, Lu DB, Pang Z, Liu QF. Vitamin C intake, circulating vitamin C and risk of stroke: a meta-analysis of prospective studies. J Am Heart Assoc. 2013;2(6):e000329. (PubMed)
60. Ye Y, Li J, Yuan Z. Effect of antioxidant vitamin supplementation on cardiovascular outcomes: a meta-analysis of randomized controlled trials. PLoS One. 2013;8(2):e56803. (PubMed)
61. Bertoia M, Albanes D, Mayne ST, Mannisto S, Virtamo J, Wright ME. No association between fruit, vegetables, antioxidant nutrients and risk of renal cell carcinoma. Int J Cancer. 2010;126(6):1504-1512. (PubMed)
62. Heinen MM, Verhage BA, Goldbohm RA, van den Brandt PA. Intake of vegetables, fruits, carotenoids and vitamins C and E and pancreatic cancer risk in The Netherlands Cohort Study. Int J Cancer. 2012;130(1):147-158. (PubMed)
63. Roswall N, Olsen A, Christensen J, Dragsted LO, Overvad K, Tjonneland A. Micronutrient intake and risk of urothelial carcinoma in a prospective Danish cohort. Eur Urol. 2009;56(5):764-770. (PubMed)
64. Goodman M, Bostick RM, Kucuk O, Jones DP. Clinical trials of antioxidants as cancer prevention agents: past, present, and future. Free Radic Biol Med. 2011;51(5):1068-1084. (PubMed)
65. Zhang S, Hunter DJ, Forman MR, et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst. 1999;91(6):547-556. (PubMed)
66. Michels KB, Holmberg L, Bergkvist L, Ljung H, Bruce A, Wolk A. Dietary antioxidant vitamins, retinol, and breast cancer incidence in a cohort of Swedish women. Int J Cancer. 2001;91(4):563-567. (PubMed)
67. Hutchinson J, Lentjes MA, Greenwood DC, et al. Vitamin C intake from diary recordings and risk of breast cancer in the UK Dietary Cohort Consortium. Eur J Clin Nutr. 2012;66(5):561-568. (PubMed)
68 Nagel G, Linseisen J, van Gils CH, et al. Dietary beta-carotene, vitamin C and E intake and breast cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). Breast Cancer Res Treat. 2010;119(3):753-765. (PubMed)
69. Roswall N, Olsen A, Christensen J, Dragsted LO, Overvad K, Tjonneland A. Micronutrient intake and breast cancer characteristics among postmenopausal women. Eur J Cancer Prev. 2010;19(5):360-365. (PubMed)
70. Liu C, Russell RM. Nutrition and gastric cancer risk: an update. Nutr Rev. 2008;66(5):237-249. (PubMed)
71. Mirvish SS, Wallcave L, Eagen M, Shubik P. Ascorbate-nitrite reaction: possible means of blocking the formation of carcinogenic N-nitroso compounds. Science. 1972;177(4043):65-68. (PubMed)
72. Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer. 2007;10(2):75-83. (PubMed)
73 Jenab M, Riboli E, Ferrari P, et al. Plasma and dietary vitamin C levels and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Carcinogenesis. 2006;27(11):2250-2257. (PubMed)
74. Banerjee S, Hawksby C, Miller S, Dahill S, Beattie AD, McColl KE. Effect of Helicobacter pylori and its eradication on gastric juice ascorbic acid. Gut. 1994;35(3):317-322. (PubMed)
75. Zhang ZW, Patchett SE, Perrett D, Katelaris PH, Domizio P, Farthing MJ. The relation between gastric vitamin C concentrations, mucosal histology, and CagA seropositivity in the human stomach. Gut. 1998;43(3):322-326. (PubMed)
76. Chuang CH, Sheu BS, Kao AW, et al. Adjuvant effect of vitamin C on omeprazole-amoxicillin-clarithromycin triple therapy for Helicobacter pylori eradication. Hepatogastroenterology. 2007;54(73):320-324. (PubMed)
77. Krajewska B, Brindell M. Urease activity and L-ascorbic acid. J Enzyme Inhib Med Chem. 2011;26(3):309-318. (PubMed)
78. Pal J, Sanal MG, Gopal GJ. Vitamin-C as anti-Helicobacter pylori agent: More prophylactic than curative- Critical review. Indian J Pharmacol. 2011;43(6):624-627. (PubMed)
79 Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control. 2010;21(11):1745-1757. (PubMed)
80. Thompson CA, Cerhan JR. Fruit and vegetable intake and survival from non-Hodgkin lymphoma: does an apple a day keep the doctor away? Leuk Lymphoma. 2010;51(6):963-964. (PubMed)
81. Kabat GC, Kim MY, Wactawski-Wende J, Shikany JM, Vitolins MZ, Rohan TE. Intake of antioxidant nutrients and risk of non-Hodgkin's Lymphoma in the Women's Health Initiative. Nutr Cancer. 2012;64(2):245-254. (PubMed)
82. Wang L, Sesso HD, Glynn RJ, et al. Vitamin E and C supplementation and risk of cancer in men: posttrial follow-up in the Physicians' Health Study II randomized trial. Am J Clin Nutr. 2014;100(3):915-923. (PubMed)
83. Song Y, Xu Q, Park Y, Hollenbeck A, Schatzkin A, Chen H. Multivitamins, individual vitamin and mineral supplements, and risk of diabetes among older U.S. adults. Diabetes Care. 2011;34(1):108-114. (PubMed)
84. Harding AH, Wareham NJ, Bingham SA, et al. Plasma vitamin C level, fruit and vegetable consumption, and the risk of new-onset type 2 diabetes mellitus: the European prospective investigation of cancer--Norfolk prospective study. Arch Intern Med. 2008;168(14):1493-1499. (PubMed)
85. Donin AS, Dent JE, Nightingale CM, et al. Fruit, vegetable and vitamin C intakes and plasma vitamin C: cross-sectional associations with insulin resistance and glycaemia in 9-10 year-old children. Diabet Med. 2016;33(3):307-315. (PubMed)
86. Kositsawat J, Freeman VL. Vitamin C and A1c relationship in the National Health and Nutrition Examination Survey (NHANES) 2003-2006. J Am Coll Nutr. 2011;30(6):477-483. (PubMed)
87. Ashor AW, Werner AD, Lara J, Willis ND, Mathers JC, Siervo M. Effects of vitamin C supplementation on glycaemic control: a systematic review and meta-analysis of randomised controlled trials. Eur J Clin Nutr. 2017;71(12):1371-1380. (PubMed)
88. Rumbold A, Ota E, Nagata C, Shahrook S, Crowther CA. Vitamin C supplementation in pregnancy. Cochrane Database Syst Rev. 2015(9):Cd004072. (PubMed)
89. Balogun OO, da Silva Lopes K, Ota E, et al. Vitamin supplementation for preventing miscarriage. Cochrane Database Syst Rev. 2016(5):Cd004073. (PubMed)
90. Hammoud AO, Bujold E, Sorokin Y, Schild C, Krapp M, Baumann P. Smoking in pregnancy revisited: findings from a large population-based study. Am J Obstet Gynecol. 2005;192(6):1856-1862; discussion 1862-1853. (PubMed)
91. Kitsantas P, Christopher KE. Smoking and respiratory conditions in pregnancy: associations with adverse pregnancy outcomes. South Med J. 2013;106(5):310-315. (PubMed)
92. Milner AD, Rao H, Greenough A. The effects of antenatal smoking on lung function and respiratory symptoms in infants and children. Early Hum Dev. 2007;83(11):707-711. (PubMed)
93. Conde-Agudelo A, Althabe F, Belizan JM, Kafury-Goeta AC. Cigarette smoking during pregnancy and risk of preeclampsia: a systematic review. Am J Obstet Gynecol. 1999;181(4):1026-1035. (PubMed)
94. Abramovici A, Gandley RE, Clifton RG, et al. Prenatal vitamin C and E supplementation in smokers is associated with reduced placental abruption and preterm birth: a secondary analysis. Bjog. 2015;122(13):1740-1747. (PubMed)
95. McEvoy CT, Schilling D, Clay N, et al. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants: a randomized clinical trial. JAMA. 2014;311(20):2074-2082. (PubMed)
96. McEvoy CT, Milner KF, Scherman AJ, et al. Vitamin C to Decrease the Effects of Smoking in Pregnancy on Infant Lung Function (VCSIP): Rationale, design, and methods of a randomized, controlled trial of vitamin C supplementation in pregnancy for the primary prevention of effects of in utero tobacco smoke exposure on infant lung function and respiratory health. Contemp Clin Trials. 2017;58:66-77. (PubMed)
97. Alzheimer's Association. 2018 Alzheimer's Disease Facts and Figures. Available at: https://www.alz.org/alzheimers-dementia/facts-figures. Accessed 6/22/18.
98. Dixit S, Bernardo A, Walker JM, et al. Vitamin C deficiency in the brain impairs cognition, increases amyloid accumulation and deposition, and oxidative stress in APP/PSEN1 and normally aging mice. ACS Chem Neurosci. 2015;6(4):570-581. (PubMed)
99. Kook SY, Lee KM, Kim Y, et al. High-dose of vitamin C supplementation reduces amyloid plaque burden and ameliorates pathological changes in the brain of 5XFAD mice. Cell Death Dis. 2014;5:e1083. (PubMed)
100. Bowman GL. Ascorbic acid, cognitive function, and Alzheimer's disease: a current review and future direction. Biofactors. 2012;38(2):114-122. (PubMed)
101. Harrison J, Rentz DM, McLaughlin T, et al. Cognition in MCI and Alzheimer's disease: baseline data from a longitudinal study of the NTB. Clin Neuropsychol. 2014;28(2):252-268. (PubMed)
102. Hansen SN, Tveden-Nyborg P, Lykkesfeldt J. Does vitamin C deficiency affect cognitive development and function? Nutrients. 2014;6(9):3818-3846. (PubMed)
103. Bowman GL, Dodge H, Frei B, et al. Ascorbic acid and rates of cognitive decline in Alzheimer's disease. J Alzheimers Dis. 2009;16(1):93-98. (PubMed)
104. Arlt S, Muller-Thomsen T, Beisiegel U, Kontush A. Effect of one-year vitamin C- and E-supplementation on cerebrospinal fluid oxidation parameters and clinical course in Alzheimer's disease. Neurochem Res. 2012;37(12):2706-2714. (PubMed)
105. Galasko DR, Peskind E, Clark CM, et al. Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Arch Neurol. 2012;69(7):836-841. (PubMed)
106. Naeini AM, Elmadfa I, Djazayery A, et al. The effect of antioxidant vitamins E and C on cognitive performance of the elderly with mild cognitive impairment in Isfahan, Iran: a double-blind, randomized, placebo-controlled trial. Eur J Nutr. 2014;53(5):1255-1262. (PubMed)
107. Reiss GR, Werness PG, Zollman PE, Brubaker RF. Ascorbic acid levels in the aqueous humor of nocturnal and diurnal mammals. Arch Ophthalmol. 1986;104(5):753-755. (PubMed)
108. Tessier F, Moreaux V, Birlouez-Aragon I, Junes P, Mondon H. Decrease in vitamin C concentration in human lenses during cataract progression. Int J Vitam Nutr Res. 1998;68(5):309-315. (PubMed)
109. Wei L, Liang G, Cai C, Lv J. Association of vitamin C with the risk of age-related cataract: a meta-analysis. Acta Ophthalmol. 2016;94(3):e170-176. (PubMed)
110. Zheng Selin J, Rautiainen S, Lindblad BE, Morgenstern R, Wolk A. High-dose supplements of vitamins C and E, low-dose multivitamins, and the risk of age-related cataract: a population-based prospective cohort study of men. Am J Epidemiol. 2013;177(6):548-555. (PubMed)
111. Rautiainen S, Lindblad BE, Morgenstern R, Wolk A. Vitamin C supplements and the risk of age-related cataract: a population-based prospective cohort study in women. Am J Clin Nutr. 2010;91(2):487-493. (PubMed)
112. Mathew MC, Ervin AM, Tao J, Davis RM. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst Rev. 2012(6):Cd004567. (PubMed)
113. Pastor-Valero M. Fruit and vegetable intake and vitamins C and E are associated with a reduced prevalence of cataract in a Spanish Mediterranean population. BMC Ophthalmol. 2013;13:52. (PubMed)
114. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136-3141. (PubMed)
115. Saag KG, Choi H. Epidemiology, risk factors, and lifestyle modifications for gout. Arthritis Res Ther. 2006;8 Suppl 1:S2. (PubMed)
116. Choi HK, Curhan G. Gout: epidemiology and lifestyle choices. Curr Opin Rheumatol. 2005;17(3):341-345. (PubMed)
117. Gao X, Curhan G, Forman JP, Ascherio A, Choi HK. Vitamin C intake and serum uric acid concentration in men. J Rheumatol. 2008;35(9):1853-1858. (PubMed)
118. Zheng Z, Harman JL, Coresh J, et al. The dietary fructose: vitamin C intake ratio is associated with hyperuricemia in African-American adults. J Nutr. 2018;148(3):419-426. (PubMed)
119. Choi HK, Gao X, Curhan G. Vitamin C intake and the risk of gout in men: a prospective study. Arch Intern Med. 2009;169(5):502-507. (PubMed)
120. Juraschek SP, Miller ER, 3rd, Gelber AC. Effect of oral vitamin C supplementation on serum uric acid: a meta-analysis of randomized controlled trials. Arthritis Care Res (Hoboken). 2011;63(9):1295-1306. (PubMed)
121. Stamp LK, Zhu X, Dalbeth N, Jordan S, Edwards NL, Taylor W. Serum urate as a soluble biomarker in chronic gout-evidence that serum urate fulfills the OMERACT validation criteria for soluble biomarkers. Semin Arthritis Rheum. 2011;40(6):483-500. (PubMed)
122. Stamp LK, O'Donnell JL, Frampton C, Drake JM, Zhang M, Chapman PT. Clinically insignificant effect of supplemental vitamin C on serum urate in patients with gout: a pilot randomized controlled trial. Arthritis Rheum. 2013;65(6):1636-1642. (PubMed)
123. Andres M, Sivera F, Falzon L, Buchbinder R, Carmona L. Dietary supplements for chronic gout. Cochrane Database Syst Rev. 2014(10):Cd010156. (PubMed)
124. Pocobelli G, Peters U, Kristal AR, White E. Use of supplements of multivitamins, vitamin C, and vitamin E in relation to mortality. Am J Epidemiol. 2009;170(4):472-483. (PubMed)
125. Roswall N, Olsen A, Christensen J, et al. Micronutrient intake in relation to all-cause mortality in a prospective Danish cohort. Food Nutr Res. 2012;56. (PubMed)
126. Harris HR, Orsini N, Wolk A. Vitamin C and survival among women with breast cancer: a meta-analysis. Eur J Cancer. 2014;50(7):1223-1231. (PubMed)
127. Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012(3):Cd007176. (PubMed)
128. Khaw KT, Bingham S, Welch A, et al. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. European Prospective Investigation into Cancer and Nutrition. Lancet. 2001;357(9257):657-663. (PubMed)
129. Goyal A, Terry MB, Siegel AB. Serum antioxidant nutrients, vitamin A, and mortality in U.S. Adults. Cancer Epidemiol Biomarkers Prev. 2013;22(12):2202-2211. (PubMed)
130. Basili S, Tanzilli G, Mangieri E, et al. Intravenous ascorbic acid infusion improves myocardial perfusion grade during elective percutaneous coronary intervention: relationship with oxidative stress markers. JACC Cardiovasc Interv. 2010;3(2):221-229. (PubMed)
131. Wang ZJ, Hu WK, Liu YY, et al. The effect of intravenous vitamin C infusion on periprocedural myocardial injury for patients undergoing elective percutaneous coronary intervention. Can J Cardiol. 2014;30(1):96-101. (PubMed)
132. Rodrigo R, Hasson D, Prieto JC, et al. The effectiveness of antioxidant vitamins C and E in reducing myocardial infarct size in patients subjected to percutaneous coronary angioplasty (PREVEC Trial): study protocol for a pilot randomized double-blind controlled trial. Trials. 2014;15:192. (PubMed)
133. Milei J, Forcada P, Fraga CG, et al. Relationship between oxidative stress, lipid peroxidation, and ultrastructural damage in patients with coronary artery disease undergoing cardioplegic arrest/reperfusion. Cardiovasc Res. 2007;73(4):710-719. (PubMed)
134. Lassnigg A, Punz A, Barker R, et al. Influence of intravenous vitamin E supplementation in cardiac surgery on oxidative stress: a double-blinded, randomized, controlled study. Br J Anaesth. 2003;90(2):148-154. (PubMed)
135. Spoelstra-de Man AME, Elbers PWG, Oudemans-van Straaten HM. Making sense of early high-dose intravenous vitamin C in ischemia/reperfusion injury. Crit Care. 2018;22(1):70. (PubMed)
136. Dingchao H, Zhiduan Q, Liye H, Xiaodong F. The protective effects of high-dose ascorbic acid on myocardium against reperfusion injury during and after cardiopulmonary bypass. Thorac Cardiovasc Surg. 1994;42(5):276-278. (PubMed)
137. Sisto T, Paajanen H, Metsa-Ketela T, Harmoinen A, Nordback I, Tarkka M. Pretreatment with antioxidants and allopurinol diminishes cardiac onset events in coronary artery bypass grafting. Ann Thorac Surg. 1995;59(6):1519-1523. (PubMed)
138. Ramos C, Brito R, Gonzalez-Montero J, et al. Effects of a novel ascorbate-based protocol on infarct size and ventricle function in acute myocardial infarction patients undergoing percutaneous coronary angioplasty. Arch Med Sci. 2017;13(3):558-567. (PubMed)
139. Valls N, Gormaz JG, Aguayo R, et al. Amelioration of persistent left ventricular function impairment through increased plasma ascorbate levels following myocardial infarction. Redox Rep. 2016;21(2):75-83. (PubMed)
140. Hemila H, Suonsyrja T. Vitamin C for preventing atrial fibrillation in high risk patients: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2017;17(1):49. (PubMed)
141. Hu X, Yuan L, Wang H, et al. Efficacy and safety of vitamin C for atrial fibrillation after cardiac surgery: A meta-analysis with trial sequential analysis of randomized controlled trials. Int J Surg. 2017;37:58-64. (PubMed)
142. Polymeropoulos E, Bagos P, Papadimitriou M, Rizos I, Patsouris E, Tauoumpoulis I. Vitamin C for the prevention of postoperative atrial fibrillation after cardiac surgery: a meta-analysis. Adv Pharm Bull. 2016;6(2):243-250. (PubMed)
143. Lagowska-Lenard M, Stelmasiak Z, Bartosik-Psujek H. Influence of vitamin C on markers of oxidative stress in the earliest period of ischemic stroke. Pharmacol Rep. 2010;62(4):751-756. (PubMed)
144. Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4:5. (PubMed)
145. Balbi ME, Tonin FS, Mendes AM, et al. Antioxidant effects of vitamins in type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetol Metab Syndr. 2018;10:18. (PubMed)
146. Khodaeian M, Tabatabaei-Malazy O, Qorbani M, Farzadfar F, Amini P, Larijani B. Effect of vitamins C and E on insulin resistance in diabetes: a meta-analysis study. Eur J Clin Invest. 2015;45(11):1161-1174. (PubMed)
147. Gillani SW, Sulaiman SAS, Abdul MIM, Baig MR. Combined effect of metformin with ascorbic acid versus acetyl salicylic acid on diabetes-related cardiovascular complication; a 12-month single blind multicenter randomized control trial. Cardiovasc Diabetol. 2017;16(1):103. (PubMed)
148. Levy AP, Friedenberg P, Lotan R, et al. The effect of vitamin therapy on the progression of coronary artery atherosclerosis varies by haptoglobin type in postmenopausal women. Diabetes Care. 2004;27(4):925-930. (PubMed)
149. Asleh R, Levy AP. Divergent effects of alpha-tocopherol and vitamin C on the generation of dysfunctional HDL associated with diabetes and the Hp 2-2 genotype. Antioxid Redox Signal. 2010;12(2):209-217. (PubMed)
150. Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250-1256. (PubMed)
151. Mann EA, Baun MM, Meininger JC, Wade CE. Comparison of mortality associated with sepsis in the burn, trauma, and general intensive care unit patient: a systematic review of the literature. Shock. 2012;37(1):4-16. (PubMed)
152. Carr AC, Rosengrave PC, Bayer S, Chambers S, Mehrtens J, Shaw GM. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit Care. 2017;21(1):300. (PubMed)
153. Pravda J. Metabolic theory of septic shock. World J Crit Care Med. 2014;3(2):45-54. (PubMed)
154. Fowler AA, 3rd, Syed AA, Knowlson S, et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014;12:32. (PubMed)
155. Zabet MH, Mohammadi M, Ramezani M, Khalili H. Effect of high-dose Ascorbic acid on vasopressor's requirement in septic shock. J Res Pharm Pract. 2016;5(2):94-100. (PubMed)
156. Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. 2017;151(6):1229-1238. (PubMed)
157. Spoelstra-de Man AME, Elbers PWG, Oudemans-Van Straaten HM. Vitamin C: should we supplement? Curr Opin Crit Care. 2018;24(4):248-255. (PubMed)
158. Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A. 1976;73(10):3685-3689. (PubMed)
159. Ohno S, Ohno Y, Suzuki N, Soma G, Inoue M. High-dose vitamin C (ascorbic acid) therapy in the treatment of patients with advanced cancer. Anticancer Res. 2009;29(3):809-815. (PubMed)
160. Chen Q, Espey MG, Krishna MC, et al. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci U S A. 2005;102(38):13604-13609. (PubMed)
161. Chen Q, Espey MG, Sun AY, et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci U S A. 2007;104(21):8749-8754. (PubMed)
162. Chen Q, Espey MG, Sun AY, et al. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci U S A. 2008;105(32):11105-11109. (PubMed)
163. Gao P, Zhang H, Dinavahi R, et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. 2007;12(3):230-238. (PubMed)
164. Kuiper C, Molenaar IG, Dachs GU, Currie MJ, Sykes PH, Vissers MC. Low ascorbate levels are associated with increased hypoxia-inducible factor-1 activity and an aggressive tumor phenotype in endometrial cancer. Cancer Res. 2010;70(14):5749-5758. (PubMed)
165. Vissers MCM, Das AB. Potential mechanisms of action for vitamin C in cancer: reviewing the evidence. Front Physiol. 2018;9:809. (PubMed)
166. Carr AC, Cook J. Intravenous vitamin C for cancer therapy - identifying the current gaps in our knowledge. Front Physiol. 2018;9:1182. (PubMed)
167. Hoffer LJ, Levine M, Assouline S, et al. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol. 2008;19(11):1969-1974. (PubMed)
168. Monti DA, Mitchell E, Bazzan AJ, et al. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS One. 2012;7(1):e29794. (PubMed)
169 Riordan HD, Casciari JJ, Gonzalez MJ, et al. A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. P R Health Sci J. 2005;24(4):269-276. (PubMed)
170. Stephenson CM, Levin RD, Spector T, Lis CG. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother Pharmacol. 2013;72(1):139-146. (PubMed)
171. Carr AC, Vissers MC, Cook JS. The effect of intravenous vitamin C on cancer- and chemotherapy-related fatigue and quality of life. Front Oncol. 2014;4:283. (PubMed)
172. Klingelhoeffer C, Kammerer U, Koospal M, et al. Natural resistance to ascorbic acid induced oxidative stress is mainly mediated by catalase activity in human cancer cells and catalase-silencing sensitizes to oxidative stress. BMC Complement Altern Med. 2012;12:61. (PubMed)
173. Hong SW, Lee SH, Moon JH, et al. SVCT-2 in breast cancer acts as an indicator for L-ascorbate treatment. Oncogene. 2013;32(12):1508-1517. (PubMed)
174. Fritz H, Flower G, Weeks L, et al. Intravenous vitamin C and cancer: a systematic review. Integr Cancer Ther. 2014;13(4):280-300. (PubMed)
175. Jacobs C, Hutton B, Ng T, Shorr R, Clemons M. Is there a role for oral or intravenous ascorbate (vitamin C) in treating patients with cancer? A systematic review. Oncologist. 2015;20(2):210-223. (PubMed)
176. Cabanillas F. Vitamin C and cancer: what can we conclude--1,609 patients and 33 years later? P R Health Sci J. 2010;29(3):215-217. (PubMed)
177. Pauling LC. Vitamin C and the Common Cold. San Francisco: W.H. Freeman; 1970.
178. Hemila H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013(1):Cd000980. (PubMed)
179. Hemila H. Vitamin C and common cold-induced asthma: a systematic review and statistical analysis. Allergy Asthma Clin Immunol. 2013;9(1):46. (PubMed)
180. Milan SJ, Hart A, Wilkinson M. Vitamin C for asthma and exercise-induced bronchoconstriction. Cochrane Database Syst Rev. 2013(10):Cd010391. (PubMed)
181. Cheng Y, Willett WC, Schwartz J, Sparrow D, Weiss S, Hu H. Relation of nutrition to bone lead and blood lead levels in middle-aged to elderly men. The Normative Aging Study. Am J Epidemiol. 1998;147(12):1162-1174. (PubMed)
182. Simon JA, Hudes ES. Relationship of ascorbic acid to blood lead levels. JAMA. 1999;281(24):2289-2293. (PubMed)
183. Lee DH, Lim JS, Song K, Boo Y, Jacobs DR, Jr. Graded associations of blood lead and urinary cadmium concentrations with oxidative-stress-related markers in the U.S. population: results from the third National Health and Nutrition Examination Survey. Environ Health Perspect. 2006;114(3):350-354. (PubMed)
184. Dawson EB, Evans DR, Harris WA, Teter MC, McGanity WJ. The effect of ascorbic acid supplementation on the blood lead levels of smokers. J Am Coll Nutr. 1999;18(2):166-170. (PubMed)
185. Abam E, Okediran BS, Odukoya OO, Adamson I, Ademuyiwa O. Reversal of ionoregulatory disruptions in occupational lead exposure by vitamin C. Environ Toxicol Pharmacol. 2008;26(3):297-304. (PubMed)
186. Carr AC, Vissers MC. Synthetic or food-derived vitamin C--are they equally bioavailable? Nutrients. 2013;5(11):4284-4304. (PubMed)
187. Johnston CS, Luo B. Comparison of the absorption and excretion of three commercially available sources of vitamin C. J Am Diet Assoc. 1994;94(7):779-781. (PubMed)
188. Austria R, Semenzato A, Bettero A. Stability of vitamin C derivatives in solution and topical formulations. J Pharm Biomed Anal. 1997;15(6):795-801. (PubMed)
189. DeRitter E, Cohen N, Rubin SH. Physiological availability of dehydro-L-ascorbic acid and palmitoyl-L-ascorbic acid. Science. 1951;113:628-631. (PubMed)
190. Davis JL, Paris HL, Beals JW, et al. Liposomal-encapsulated ascorbic acid: influence on vitamin C bioavailability and capacity to protect against ischemia-reperfusion injury. Nutr Metab Insights. 2016;9:25-30. (PubMed)
191. Massey LK, Liebman M, Kynast-Gales SA. Ascorbate increases human oxaluria and kidney stone risk. J Nutr. 2005;135(7):1673-1677. (PubMed)
192. Traxer O, Huet B, Poindexter J, Pak CY, Pearle MS. Effect of ascorbic acid consumption on urinary stone risk factors. J Urol. 2003;170(2 Pt 1):397-401. (PubMed)
193. Auer BL, Auer D, Rodgers AL. The effect of ascorbic acid ingestion on the biochemical and physicochemical risk factors associated with calcium oxalate kidney stone formation. Clin Chem Lab Med. 1998;36(3):143-147. (PubMed)
194. Liebman M, Chai WW, Harvey E, Boenisch L. Effect of supplemental ascorbate and orange juice on urinary oxalate. Nutr Res. 1997;17(3):415-425.
195. Wandzilak TR, D'Andre SD, Davis PA, Williams HE. Effect of high dose vitamin C on urinary oxalate levels. J Urol. 1994;151(4):834-837. (PubMed)
196. Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of the intake of vitamins C and B6, and the risk of kidney stones in men. J Urol. 1996;155(6):1847-1851. (PubMed)
197. Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Intake of vitamins B6 and C and the risk of kidney stones in women. J Am Soc Nephrol. 1999;10(4):840-845. (PubMed)
198. Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol. 2004;15(12):3225-3232. (PubMed)
199. Thomas LD, Elinder CG, Tiselius HG, Wolk A, Akesson A. Ascorbic acid supplements and kidney stone incidence among men: a prospective study. JAMA Intern Med. 2013;173(5):386-388. (PubMed)
200. Natural Medicines. Vitamin C/Professional handout/Interactions with drugs. Available at: https://naturalmedicines.therapeuticresearch.com. Accessed 7/2/18.
201. Hendler SS, Rorvik DM. PDR for Nutritional Supplements. 2nd ed. Montvale: Thomson Reuters; 2008.
202 Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345(22):1583-1592. (PubMed)
203. Collins R, Peto R, Armitage J. The MRC/BHF Heart Protection Study: preliminary results. Int J Clin Pract. 2002;56(1):53-56. (PubMed)
204. Lee SH, Oe T, Blair IA. Vitamin C-induced decomposition of lipid hydroperoxides to endogenous genotoxins. Science. 2001;292(5524):2083-2086. (PubMed)
205. Podmore ID, Griffiths HR, Herbert KE, Mistry N, Mistry P, Lunec J. Vitamin C exhibits pro-oxidant properties. Nature. 1998;392(6676):559. (PubMed)
206. Carr A, Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? Faseb J. 1999;13(9):1007-1024. (PubMed)
207. Brubacher D, Moser U, Jordan P. Vitamin C concentrations in plasma as a function of intake: a meta-analysis. Int J Vitam Nutr Res. 2000;70(5):226-237. (PubMed)
208. Michels AJ, Joisher N, Hagen TM. Age-related decline of sodium-dependent ascorbic acid transport in isolated rat hepatocytes. Arch Biochem Biophys. 2003;410(1):112-120. (PubMed)
How Much Vitamin C Can Cause Kidney Stones
Source: https://lpi.oregonstate.edu/book/export/html/148

0 komentar:
Posting Komentar